Original
Detrimental Responses of the Properties in Rat Soleus Muscle to Passive Continuous Stretch
Hisashi Kato1,3,4,*, Daishin Ueno1,*, Takashi Ohira4,5, Fuminori Kawano4,6, Katsumasa Goto4,7, Hideki Ohno8, Tetsuya Izawa1-4, Yoshinobu Ohira1-4
1Faculty and 2Graduate School of Health & Sports Science, Research Center for 3Adipocyte and Muscle Science and 4Space and Medical Sciences, Doshisha University
5Division of Aerospace Medicine, Department of Cell Physiology, Jikei University School of Medicine
6Graduate School of Health Sciences, Matsumoto University, Matsumoto City
7School of Health Sciences, Toyohashi SOZO University
8Social Medical Corporation Foundation “Yamatokai”
*Equally contributed authors
ABSTRACT
Responses of the properties in rat soleus muscle to continuous stretch for 10 days were studied in male Wistar Hannover rats. Wet weights and fiber cross-sectional areas were increased following stretch keeping the anterior angle of ankle joint at 〜30°, equivalent to the level when the rats maintain during rest in a prone position. However, such phenomena were related to a trend that percent content of water per wet weight was increased due to edema, associated with decrease in protein content. Fiber type composition and the protein expression of slow myosin heavy chain remained stable. Succinate dehydrogenase activity, analyzed in single fibers, and citrate synthase activity and content of cytochrome c oxidase IV, analyzed in whole homogenates, were lowered. Expressions of apoptosis-related parameters, such as caspase-3 protein and BAX/BLC2 ratio, where BCL2 and BAX are B-cell leukemia/lymphoma 2 and BCL2-associated X, were increased. And expressions of BCL2 and BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 were lowered. But the expressions of autophagy-related parameters, such as LC3-II/LC3-I ratio, where LC3 is microtubule-associated protein 1A/1B light chain 3, were lowered. It was indicated that the chronic stretch induced detrimental effects, damage and apoptosis, on the morphological and metabolic properties of soleus. But it was suggested that the autophagy function may not be efficient enough to inhibit the damage, associated with apoptosis.
(Received:5 January, 2018 Accepted:12 January, 2018)
Key words:continuous stretch of rat soleus, fiber size and phenotype, apoptosis, autophagy
INTRODUCTION
Removal of weight-bearing activity induces a drastic effect on the skeletal muscles responsible for maintenance of posture and ground support, i.e., muscles composed predominantly of slow-twitch fibers. One of the pronounced responses is decrease (atrophy) of fiber cross-sectional area (CSA), accompanied by fiber-phenotype transition from slow to fast phenotype, especially in soleus11,17)and adductor longus muscle16). Decrease in the percent composition of pure slow fibers and increase in hybrid fibers, which express both slow and fast myosin heavy chain (MHC), are generally induced. Furthermore, mitochondrial enzyme activities in whole muscle homogenates15)are also lowered following chronic gravitational unloading. The specific activities of succinate dehydrogenase (SDH) in atrophied muscle fibers are often maintained or even elevated11,16,17), although the total activities in whole muscle fiber area are lower than normal controls16,17). These results indicate that the unloading-related decreases of fiber CSA and SDH activity are relatively parallel or the degree of atrophy is slightly greater.
One of the major causes for such changes in skeletal muscles is the inhibition of mechanical stress, although neural activity is also decreased during gravitational unloading7,11,13). Thus, it is essential to develop some suitable countermeasures for prevention of atrophy. Beneficial effects of stretch, which could be one of the methods as the countermeasure, on the properties of skeletal muscle are reported. Hypertrophy was induced in chicken wing muscles following passive stretch, even though the muscle activity estimated by recording electromyogram remained unchanged5). Oxidative enzyme activities were also increased in fast-twitch muscle, patagialis. But such adaptation was not noted in slow-tonic muscle, anterior latissimus dorsi, in response to stretch. The cause of such discrepancy is unclear.
It was reported that adult rats spend 〜78% of time at rest in a recumbent prone position18). The anterior angle of ankle joint during rest on the floor is 〜30°, and the sarcomeres in soleus muscle fibers were passively stretched at 〜3.03 µm (vs. 2.5 µm normal resting length)7). However, it is still unclear how the properties of slow-twitch soleus and muscle fibers respond to passive stretch. Therefore, the current study was performed to investigate the responses to passive chronic stretch at the length equivalent to the level, which is maintained 〜78% of time daily by fixing the ankle joint at 〜30°.
MATERIAL & METHODS
Experimental design and animal care
The protocol of this experiment was approved by the Animal-Care Committee at Doshisha University (No. A17010). Twelve male Wistar Hannover rats (Shimizu Lab Supplies, Japan) with the age of 8 weeks old at the beginning of experiment were used. The rats were randomly separated into the cage control and hindlimb suspension groups (n=6 each). The left ankle joint of hindlimb suspension group was fixed at 〜30° using plaster cast under anesthesia with i.p. injection of sodium pentobarbital (5 mg/100 g body weight) and the contralateral joint was kept free. The ankle joints of both limbs in the cage controls were kept free.
Hindlimb suspension was performed for 10 days, as was described elsewhere7,16). The contact of hindlimbs with the floor and/or cage wall was avoided. During this period, the rats were able to eat solid diet and drink water freely by using their forelimbs. The left soleus muscles in all rats were sampled after 10 days. Anesthesia with i.p. injection of sodium pentobarbital was performed, when the rat was still hindlimb-suspended to avoid the effects of sudden mechanical loading on the floor. The muscles then were wet weighed. The muscles were stretched gently to near optimum in vivo length, pinned on a cork, and frozen in isopentane cooled with liquid nitrogen. The samples were then stored at −80℃ until analyses.
Cross-sectional analyses of muscle fibers
Fiber phenotypes and cross-sectional areas: Mid-portion of frozen muscle was cut on dry ice, mounted perpendicularly on a cork using optimum cutting temperature (OCT) compound, and frozen in liquid nitrogen. The remaining portions were stored at −80℃ for whole homogenate analyses. Standard immunohistochemical staining was performed in the serial cross-sections (10-µm thickness), cut in a cryostat maintained at −20℃. Muscle sections were fixed with 4% paraformaldehyde for 15 min. Blocking was performed using 10% donkey serum (Sigma-Aldrich, St. Louis, MO, USA) in 1% triton X-100 mixed with 0.1 M phosphate-buffered saline (PBS) for 1 hr. The expression of MHC in individual fiber was analyzed by using mouse monoclonal antibodies {NCL-MHCs (Leica, Nußloch, Germany) or NCL-MHCf (Leica)} specific to slow (type I) or fast (type II) MHC isoforms, as described previously16. In addition, laminin was stained by using rabbit anti-laminin antibodies (Sigma-Aldrich) to measure the size of CSA of fibers. Primary antibodies were diluted 1:50 with 0.1 M PBS, containing 5% donkey serum and 0.3% triton X-100, and reacted with muscle sections at 4℃. Alexa fluor 594 donkey anti-mouse IgG antibody and Alexa fluor 488 donkey anti-rabbit IgG antibody (Invitrogen, Tokyo, Japan) were used as the second antibodies. Second antibodies were diluted 1:400 with 0.1M PBS containing 5% donkey serum and 0.1% triton X-100 and reacted with muscle sections overnight at 4℃. Finally, the sections were air-dried and cover-slipped. Between each step, muscle sections were washed by immersing in 0.1M PBS for 5-20 min twice.
Numbers of primary antibody positive fibers were counted in the deep region of soleus muscle. Fiber phenotypes were classified as pure slow or fast type, or hybrid type expressing both slow and fast MHC. Fibers in the same area of muscle cross-section were analyzed for CSA and phenotype, as well SDH activity, in the each muscle sample16,17). These parameters in at least 100 fibers were matched, as is stated below.
Enzyme activities:The activities of SDH, an oxidative enzyme, were determined immuno- histochemically, as described previously16,17). The stained images in whole area of fibers were digitized as grey-level pictures. Arrays of picture elements were quantified to grey levels that were then converted automatically to an optical density (OD). The OD level was divided by the incubation time and the enzyme activities were expressed as the specific activity (ΔOD/min). The OD level of each fiber was matched with MHC expression and CSA level.
Biochemical analyses in whole homogenate of muscle
Citrate synthase activity:The remaining portions of soleus muscles were homogenized using RIPA Lysis kit (ATTO, Tokyo, Japan). The homogenate was mixed with assay buffer consisting 100 mM Tris-HCl, pH 8.0, 1 mM 5,5´-dithiobis-2-nitrobenzoic acid (DTNB), 3 mM acetyl-CoA, and 5 mM oxaloacetate. Citrate synthase activity was determined in duplicate as previously described27). Absorbance at 412 nm was measured using U-1900 spectrophotometer (HITACHI, Tokyo, Japan). Background was determined using assay buffer without 5 mM oxaloacetate and subtracted from all sample values. Citrate synthase activity was calculated as µmol/min/g wet weight and nmol/min/mg protein. Total protein was measured by Bradford assay1). Calculation of total protein in whole muscle was also used for the estimation of percent content of protein and water relative to the wet weight of muscle15).
Western blot analyses:The whole homogenates, utilized for the analyses of slow MHC (MHC-slow), cytochrome c, cytochrome c oxidase IV (COX IV), microtubule-associated protein 1A/1B light chain 3 (LC3), p62, B-cell leukemia/lymphoma 2 (BCL2) and BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3), caspase-3, and BCL2-associated X (BAX), were centrifuged at 15,000 g for 4 min. And the supernatants were then boiled in sample buffer8)for 2 min, adjusted to a final protein concentration at 1 mg/mL according to the method of Bradford1). After separation on 6-15% sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) gels, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (ATTO), which were blocked for 60 min with Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) and 5% skim milk. The membranes were incubated with the following primary antibodies:α-tubulin (ab176560, Abcam, Cambridge, UK), cytochrome c (MAB897, Novus Biologicals, Littleton, CO, USA), MHC-slow (M8421, Sigma-Aldrich), COX IV (ab14744, Abcam), LC3 (PM036, MBL, Nagoya, Japan), p62 (#5114, Cell Signaling Technology, Inc., Danvers, MA, USA), BNIP3 (ab10433, Abcam), BCL2 (SAB4500003, Sigma-Aldrich), BAX (#2772, Cell Signaling Technology), and caspase-3 (#9662, Cell Signaling Technology). For the analyses of all proteins, 5-µg proteins were applied to the gel, respectively. Subsequently, membranes were incubated for 60 min with 1:2,500 dilutions of anti-rabbit or anti-mouse immunoglobulin G (GE Healthcare, Buckinghamshire, UK). Bands for specific proteins were detected using ECL Prime system (GE Healthcare) and quantified on ChemiDocTM MP system (Bio-Rad). Protein abundance was normalized to the level of α-tubulin.
Statistical analyses
All data are presented as mean±SEM. Significant differences among groups were determined using unpaired t-test. Significant differences were accepted at the level of p<0.05.
RESULTS
Morphological properties
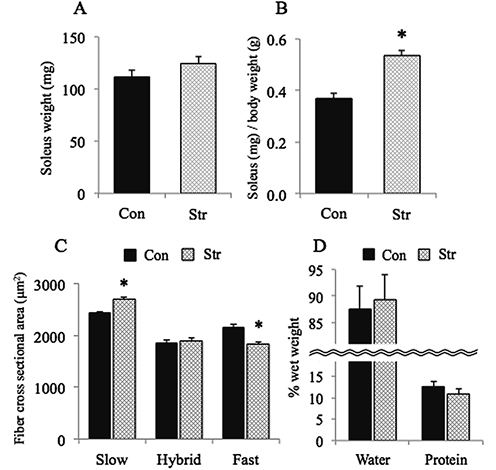
The mean body weight in the cage control group increased during 10 days of experimental period (p<0.05, data not shown). The mean body weight in the hindlimb-suspended groups was 10% lower than the pre-experimental level and 21% lower than the age-matched cage control rats, respectively (p<0.05). The absolute weight of stretched soleus tended to be 12% greater than that of cage controls (p>0.05, Fig. 1A). The weight of stretched muscle, relative to body weight, was 44% greater than the age-matched cage control rats (p<0.05, Fig. 1B).
The mean CSA of fibers expressing pure slow MHC in the stretched muscle was 11% greater than that in the age-matched cage control (p<0.05, Fig. 1C). The CSA of hybrid fibers was similar to that in the cage controls. As for the fibers expressing pure fast MHC, the mean CSA were 16% less than that in the cage controls (p<0.05). The percent content of water relative to the wet weight in the stretched muscle tended to be greater and that of total protein tended to be less than cage controls (p>0.05, Fig. 1D).
Phenotype and mitochondrial metabolic properties
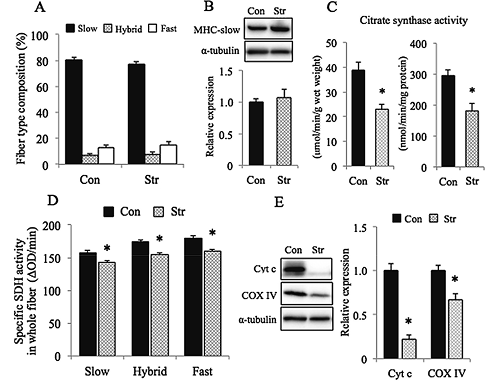
Continuous stretch of muscle did not influence the fiber type composition (Fig. 2A) and the protein expression of MHC-slow, analyzed in muscle homogenate (Fig. 2B). However, detrimental effects of stretch were noted in the mitochondrial metabolic properties. Specific activity of SDH (Fig. 2D), analyzed in the cross section of fibers, citrate synthase activity (Fig. 2C), and protein contents of cytochrome c and COX IV (Fig. 2E), analyzed in muscle homogenate of the stretched muscle, were significantly less than cage controls (p<0.05).
Autophagy-related properties
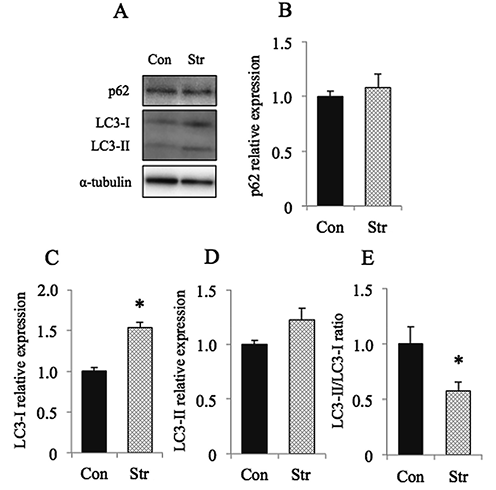
Figure 3 shows the responses of autophagy-related parameters. The expression of p62 was not influenced by stretch (Fig. 3B). But the expression of LC3-I was significantly elevated in response to stretch (p<0.05, Fig. 3C). That of LC3-II tended to be increased also, but insignificantly (p>0.05, Fig. 3D). And the ratio of the expression, LC3-II/LC3-I, decreased significantly following stretch (p<0.05, Fig. 3E).
Apoptosis-related properties
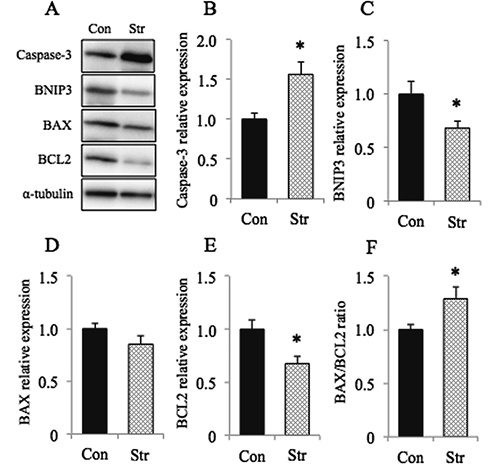
As for the apoptosis-related parameters, the expression of caspase-3 protein was increased following stretch (p<0.05, Fig. 4B). The expression of BNIP3 was significantly lowered, on the contrary (p<0.05, Fig. 4C). Even though the decrease of BAX protein expression was not statistically significant (p>0.05, Fig. 4D), the expression of BCL2 decreased significantly (p<0.05, Fig. 4E). And the ratio, BAX/BCL2, was elevated, on the contrary (p<0.05, Fig. 4F).
 |
| Figure 1 |
Absolute (A) and relative (to body weight) weight of soleus muscle (B), and cross-sectional area of soleus muscle fibers expressing various types of myosin heavy chain (MHC, C). D:Percent content of water and total protein per wet weight of muscle. Mean±SEM. Con:cage control (n=6) and Str: stretched muscle during hindlimb suspension with fixation of ankle joint at 〜30° (n=6). Slow and Fast: fibers expressing pure slow or fast MHC, Hybrid: fibers co-expressing slow and fast MHC. *:Significantly different from Con at p<0.05. |
 |
| Figure 2 |
A:Fiber type composition (%) of soleus muscle after 10 days of cage housing or stretch of muscle. B: Expression of slow type MHC (MHC-slow). The expression level of MHC-slow protein relative to Con was normalized by α-tubulin level. C:Citrate synthase activity, analyzed in whole homogenates. D: Specific activity of succinate dehydrogenase (SDH), analyzed in the cross-section of muscle fibers. E: Expression of cytochrome c (Cyt c) and cytochrome c oxidase IV (COX IV) proteins, analyzed in whole homogenates. Mean±SEM. n=6 in each group. *: Significantly different from Con at p<0.05. See Figure 1 for other abbreviations. |
 |
| Figure 3 |
A:Representative staining patterns of autophagy-related proteins, p62, microtubule-associated protein 1 light chain 3-I (LC3-I), and LC3-II, as well as α-tubulin. And the mean relative expressions of p62 (B), LC3-I (C), LC3-II (D), and the ratio of LC3-II/LC3-I (E) are also shown. Mean±SEM. *:Significantly different from Con at p<0.05. See Figure 1 for other abbreviations. |
 |
| Figure 4 |
A:Representative staining patterns of apoptosis-related proteins, caspase-3, B-cell leukemia/lymphoma 2 (BCL2), and BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3), BCL2-associated X (BAX), and α-tubulin. And the mean relative expressions of caspase-3 (B), BNIP3 (C), BAX (D), BCL2 (E), and the ratio of BAX/BCL2 (F) are also shown. Mean±SEM. *: Significantly different from Con at p<0.05. See Figure 1 for other abbreviations. |
DISCUSSION
The purpose of the present study was to investigate the adaptation of antigravity muscle, soleus, to chronic passive stretch at the length equivalent to the level at rest in a prone position on the floor7,15). As the results, damage-related increase of muscle mass and apoptosis, and decrease of mitochondrial metabolic parameters were noted. But clear responses were not noted in the autophagy-related parameters.
Morphological properties
Muscle weight, relative to body weight, and CSA in fibers expressing slow MHC were increased after 10 days of continuous stretch during hindlimb suspension. However, data suggested that such responses may be related to edema due to damage-associated increase of water content. Such responses were opposite from the previously reported results that the percent water content decreased due to the greater loss of absolute water content caused by fluid shift from hindlimbs toward the head during head-down tilt hindlimb suspension15). Even though the absolute content of protein was also decreased, its relative content per wet weight was increased due to greater loss of water, on the contrary15). However, it is unclear why the response of CSA in fibers expressing hybrid (〜7%) and pure fast MHC (〜12%) were different from that in slow fibers expressing approximately 81%.
Detrimental responses
Anti-gravitational muscles with greater mitochondrial metabolic properties are mainly composed of fibers expressing slow type MHC11,13,16,17,20). And it is well-reported that both decrease of the specific activities of mitochondrial enzymes and shift of fiber phenotype from slow to fast type are generally induced following gravitational unloading. However, opposite responses were induced in response to chronic stretch in the present study. The composition of fiber phenotype remained unchanged. But the activities of citrate synthase and SDH, as well as protein content of cytochrome c and COX IV, significantly decreased, indicating that detrimental effects were induced following chronic stretch. The data indicate that the mechanism responsible for the regulation of fiber phenotype and mitochondrial metabolic properties are different, even though they are generally closely correlated11,13,17,20).
The results clearly indicated that the level of caspase-3 protein was elevated and that of BNIP3 and BCL2 was decreased following chronic stretch of muscle vs. cage controls (Fig. 4), suggesting that the reduction of the parameters involved in mitochondrial metabolism seen in the chronically stretched muscle may be related to damage and/or apoptosis3,4,26). Stretch-related elevation of BAX/BCLI2 ratio also indicates the increased sensitivity to apoptosis. Data also suggested that such responses may be related to edema due to the damage-associated increase of water content vs. normal controls. It is speculated that the functional properties of muscle in the stretched muscles, with lowered mitochondrial metabolic properties, may be subnormal, even though the muscle weight and size of slow fibers were even increased.
It was reported that increased mobilization of anti-gravitational slow-twitch muscle, soleus, during 1-month ambulation recovery following 4-month bedrest caused fiber damage in human subjects21,29). The bedrest-related decrease of maximal tension development of single fibers, sampled by biopsy, recovered toward the pre-bedrest level (93%) after 1-month recovery29). But the fiber cross-sectional areas (CSAs) were increased to more than the pre-bedrest levels (+21%, p<0.05), indicating that the increased CSA was not closely related to the function of fibers21). Hindlimb suspension of rats caused plantar flexion of ankle joints to 〜160° and passive shortening of soleus muscle fibers to 〜66% and sarcomeres to 〜2.05 µm, which inhibited tension development in the slack muscle7). After 2 weeks of suspension, total number of sarcomeres was decreased by 62%, due to remodeling. And sarcomeres were over-stretched to 〜3.33 µm, when the rats were returned to cage and prone position was maintained. Similar remodeling of sarcomeres might be also induced in human soleus, since the ankle joints are plantar-flexed during bedrest. Thus, responses seen in human soleus fibers after 1-month recovery from 4-month bedrest21) may be related to sudden over-stretch and increased contractile activity during ambulation recovery.
It was also reported that continuous stretch of frog sartorius muscle to 110% of resting length during 5 days of organ culture resulted in marked deterioration with decreases in total protein, citrate synthase activity, and contractile force19). Such responses may be related to the experimental model, in which muscle lost the properties to readapt to stretch through afferent input, because the muscle was completely denervated. Although the innervation was kept intact in the present study, similar effects were induced by stretch. Thus, these results suggest that continuous stretch may cause detrimental effects on the properties of skeletal muscle regardless of innervation, even though occasional stretch followed by relaxation may be beneficial.
Hemeprotein, cytochrome c, is important for mitochondria-dependent apoptosis in rat skeletal muscle28). Apoptotic activity in rat soleus was significantly higher than that in the cage control group after 14 days of hindlimb suspension22). In the early stage of apoptosis, mitochondrial cytochrome c was released into the cytosol, and subsequently caused apoptosis by activating caspases10). It was reported that cytochrome c in whole cell also decreased due to probably protein degradation after depletion of mitochondrial cytochrome c2). Roles of oxidative stress as a key regulator of cell signaling pathways, which cause proteolysis and atrophy in skeletal muscle following chronic inactivity, were reviewed by Powers et al.23,24,25). They stated that one of the major pathways is related to caspase-3.
Increased expression of caspase 3 and decreased expression of BNIP3 and BCL2, associated with increase in BAX/BCL2 ratio, were observed in tibialis anterior (TA) muscle of mice following immobilization of ankle joint at a plantar-flexed position6). Even though TA is a fast-twitch muscle, the muscle is passively stretched by plantar-flexion of ankle joint15). Therefore, these results may be closely related to the stretch of TA, as was seen in the present study. Thus, the results shown in Figure 4 clearly indicated that passive continuous stretch caused apoptosis in slow-twitch soleus.
Beneficial roles of autophagy for adaptation of skeletal muscle and/or maintenance of muscle mass were reported9,12,14). Kang et al.6) reported that the expression of p62 and LC3-I, and LC3II/LC3-I ratio were increased and cytochrome c oxidase activity was decreased in mouse TA following ankle joint immobilization keeping the muscle at a stretched length. Mitochondrial enzyme activities were lowered and expression of LC3-I was increased similarly in the present study. But p62 did not change and LC3-II/LC3-I ratio was decreased, on the contrary. Thus, the results obtained in the current study may suggest that the autophagy function may not be efficient enough to inhibit the damage, associated with apoptosis, caused by forced continuous stretch in slow-twitch soleus muscle.
In conclusion, adaptation of antigravity muscle, soleus, to 10 days of chronic stretch at the length equivalent to the level at rest in a prone position on the floor was studied in male Wistar Hannover rats. Wet weight and fiber CSA were increased in muscle kept at a stretched length. However, edema-related increase of muscle mass and apoptosis, and decrease of mitochondrial metabolic properties were induced. But, clear responses were not noted in the autophagy-related parameters. It was suggested that continuous stretch caused detrimental responses in soleus muscle.
AUTHOR CONTRIBUTIONS
HK, DU, and YO performed the experiment. HK, DU, TO, FK, KG, and HO contributed to the analyses of data. HK, DU, TI, and YO contributed to all aspects in this study, including study design, drafting of the manuscript and executing the final revisions. All authors approved the final manuscript prior to publication.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
This study was supported, in part, by a grant-in-aid from Harris Science Institute, Doshisha University (YO). The funder had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
REFERENCES
| 1) |
Bradford, M.M.:A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248-254, 1976. |
| 2) |
Chen, Q., Gong, B. and Almasan, A.:Distinct stages of cytochrome c release from mitochondria:evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ., 7, 227-233, 2000. |
| 3) |
Dupont-Versteegden, E.E.:Apoptosis in skeletal muscle and its relevance to atrophy. World J. Gastroenterol., 12, 7463-7466, 2006. |
| 4) |
Guglielmi, V., Vattemi, G., Chignola, R., Chiarini, A., Marini, M., Dal Prà, I., Di Chio, M., Chiamulera, C., Armato, U. and Tomelleri, G.:Evidence for caspase-dependent programmed cell death along with repair processes in affected skeletal muscle fibres in patients with mitochondrial disorders. Clin. Sci., 130, 167-181, 2016. |
| 5) |
Holly, R.G., Barnett, J.G., Ashmore, C.R., Taylor, R.G. and Molé, P.A.:Stretch-induced growth in chicken wing muscles: a new model of stretch hypertrophy. Am. J. Physiol., 238, C62-C71, 1980. |
| 6) |
Kang, C., Yeo, D. and Ji, L.L.:Muscle immobilization activates mitophagy and disrupts mitochondrial dynamics in mice. Acta Physiol., 218, 188-197, 2016. |
| 7) |
Kawano, F., Ishihara, A., Stevens, J.L., Wang, X.D., Ohshima, S., Horisaka, M., Maeda, Y., Nonaka, I. and Ohira, Y.:Tension- and afferent input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am. J. Physiol. Regul. Integr. Comp. Physiol., 287, R76-R86, 2004. |
| 8) |
Laemmli, U.K.:Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685, 1970. |
| 9) |
Lira, V.A., Okutsu, M., Zhang, M., Greene, N.P., Laker, R.C., Breen, D.S., Hoehn, K.L. and Yan, Z.:Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J., 27, 4184-4193, 2013. |
| 10) |
Liu, X., Kim, C.N., Yang, J., Jemmerson, R. and Wang, X.: Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell, 86, 147-157, 1996. |
| 11) |
Martin, T.P., Edgerton, V.R. and Grindeland, R.E.:Influence of spaceflight on rat skeletal muscle. J. Appl. Physiol., 65, 2318-2325, 1988. |
| 12) |
Masiero, E., Agatea, L., Mammucari, C., Blaauw, B., Loro, E., Komatsu, M., Metzger, D., Reggiani, C., Schiaffino, S. and Sandri, M.:Autophagy is required to maintain muscle mass. Cell Metabol., 10, 507-515, 2009. |
| 13) |
Miu, B., Martin, T.P., Roy, R.R., Oganov, V., Ilyina-Kakueva, E., Marini, J.F., Leger, J.J., Bodine-Fowler, S.C. and Edgerton, V.R.:Metabolic and morphologic properties of single muscle fibers in the rat after spaceflight, Cosmos 1887. FASEB J., 4, 64-72, 1990. |
| 14) |
Mizushima, N. and Yoshimori, T.:How to interpret LC3 immunoblotting. Autophagy, 3, 542-545, 2007. |
| 15) |
Ohira, M., Hanada, H., Kawano, F., Ishihara, A., Nonaka, I. and Ohira, Y.:Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn. J. Physiol., 52, 235-245, 2002. |
| 16) |
Ohira, T., Terada, M., Kawano, F., Nakai, N., Ogura, A. and Ohira, Y.:Region-specific responses of adductor longus muscle to gravitational load-dependent activity in Wistar Hannover rats. PLoS ONE, 6, e21044, 2011. |
| 17) |
Ohira, Y., Jiang, B., Roy, R.R., Oganov, V., Ilyina-Kakueva, E., Marini, J.F. and Edgerton, V.R.:Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J. Appl. Physiol., 73, 51S-57S, 1992. |
| 18) |
Ohira, Y., Kawano, F., Roy, R.R. and Edgerton, V.R.: Metabolic modulation of muscle fiber properties unrelated to mechanical stimuli. Jpn. J. Physiol., 53, 389-400, 2003. |
| 19) |
Ohira, Y., Ohira, M., Chen, M. and Holloszy, J.O.:Organ culture of frog muscle:maintenance of mass, enzyme levels, and contractile force. J. Appl. Physiol., 67, 466-471, 1989. |
| 20) |
Ohira, Y., Yasui, W., Kariya, F., Wakatsuki, T., Nakamura, K., Asakura, T. and Edgerton VR.:Metabolic adaptation of skeletal muscles to gravitational unloading. Acta Astronaut, 33, 113-117, 1994. |
| 21) |
Ohira, Y., Yoshinaga, T., Ohara, M., Nonaka, I., Yoshioka, T., Yamashita-Goto, K., Shenkman, B.S., Kozlovskaya, I.B., Roy, R.R. and Edgerton, V.R.:Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol., 87, 1776-1785, 1999. |
| 22) |
Oishi, Y., Ogata, T., Yamamoto, K.I., Terada, M., Ohira, T., Ohira, Y., Taniguchi, K. and Roy, R.R.:Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol., 192, 381-395, 2008. |
| 23) |
Powers, S.K., Duarte, J., Kavazis, A.N. and Talbert, E.E.: Reactive oxygen species are signaling molecules for skeletal muscle adaptation. Exp. Physiol., 95, 1-9, 2010. |
| 24) |
Powers, S.K., Kavazis, A.N. and DeRuisseau, K.C.: Mechanisoms of disuse muscle atrophy:role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol., 288, R337-R344, 2005. |
| 25) |
Powers, S.K., Kavazis, A.N. and McClung, J.M.:Oxidative stress and disuse muscle atrophy. J. Appl. Physiol., 102, 2389-2397, 2007. |
| 26) |
Schwartz, L.M.:Atrophy and programmed cell death of skeletal muscle. Cell Death Differ. 15, 1163-1169, 2008. |
| 27) |
Srere, P.A.:Citrate synthase. Methods Enzymol., 13, 3-5, 1969. |
| 28) |
Tamilselvan, J., Jayaraman, G., Sivarajan, K. and Panneerselvam, C.:Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats:role of carnitine and lipoic acid. Free Radic. Biol. Med., 43, 1656-1669, 2007. |
| 29) |
Yamashita-Goto, K., Okuyama, R., Honda, M., Kawasaki, K., Fujita, K., Yamada, T., Nonaka, I., Ohira, Y. and Yoshioka, T.: Maximal and submaximal forces of slow fibers in human soleus after bed rest. J. Appl. Physiol., 91, 417-424, 2001. |
Address for correspondence:Yoshinobu Ohira, Ph.D.
Graduate School of Health and Sports Science, Doshisha University
Tatara Miyakodani 1-3, Kyotanabe City, Kyoto 610-0394, Japan
E-mail:yohira@mail.doshisha.ac.jp



