原著
鼓膜温測定によるメニエール病の評価,および気象との関連性について
北島 尚治1,2,北島 明美1,3,北島 清治1
1北島耳鼻咽喉科医院
2東京医科大学耳鼻咽喉科
3聖マリアンナ医科大学耳鼻咽喉科
Evaluation of Meniere’s Disease Using Tympanic Membrane Temperature and Relevance Between Tympanic Membrane Temperature and Weather
Naoharu Kitajima1,2, Akemi Sugita-Kitajima1,3, Seiji Kitajima1
1Kitajima ENT Clinic
2Department of Otolaryngology, Tokyo Medical University
3Department of Otolaryngology, St. Marianna University School of Medicine
ABSTRACT
Tympanic membrane temperature (TMT) is generally used as an indicator of brain temperature in thermoregulatory studies in humans. Bilateral differences in TMT, however, have been reported in a patient with vertigo. This prompted us to evaluate TMT bilaterally in 11 Meniere’s disease patients and 8 healthy volunteers. To determine whether TMT is affected by weather, we measured and compared TMT and concurrent weather details in all subjects. We also calculated percent TMT asymmetry (%TMTA), which is the ratio of TMT of the affected ear to that of the unaffected ear. In healthy volunteers, the mean %TMTA±SD were −0.07±0.28 (right-left) or 0.07±0.28 (left-right). The mean %TMTA±SD of patients was 0.11±0.59. The mean %TMTA during the paroxysmal period was 0.19±0.62, whereas that during the interictal period was 0.10±0.58. The mean %TMTA of the patients during the paroxysmal period was significantly higher than that of healthy volunteers. Changes in the weather did not affect %TMTA. Our study provides evidence to support the practice of measuring TMT to evaluate Meniere’s disease. Since measuring TMT is easy and non-invasive, it would be a useful method also for patients in their self-management of vertigo.
(Received:5 February, 2015 Accepted:13 January, 2016)
Key words:Tympanic membrane temperature, Meniere’s disease, endolymphatic sac, autonomic dysfunction, weather
I. INTRODUCTION
Meniere’s disease is an inner ear disorder clinically characterized by episodic vertigo with fluctuating hearing loss and tinnitus. Its clinical course is cyclical and unpredictable. The etiology and mechanisms underlying Meniere’s disease remain unclear, but its pathophysiology involves endolymphatic hydrops. Several studies have suggested that Meniere’s disease is influenced by weather conditions, specifically cold fronts17,24).
Tympanic membrane temperature (TMT) is used widely as an indicator of brain temperature in thermoregulatory studies in humans and was first reported by Bezinger as a possible locus for accurate recording of deep core temperature3). Bilateral differences in TMT were reported in a patient with vertigo18,25). This might be related to alterations in cerebral blood flow due to autonomic dysfunction18,25).
Measuring TMT is easy and non-invasive. Thus, we reasoned that patients with Meniere’s disease would benefit by monitoring their own TMT with a TMT thermometer. The present study was undertaken to clarify the relationship between TMT and the symptoms of Meniere’s disease. We also investigated the relationship between TMT and weather conditions, such as atmospheric pressure, temperature, and humidity.
II. METHODS
Eleven patients with Meniere’s disease (3 males and 8 females;age:35-83 years;mean±SD:59±16 years) and 8 healthy volunteers (1 male and 7 females; age:36-70 years;mean ± SD:52 ± 11 years) participated in this study. The study was conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the prior written consent of the participants. All procedures were approved by the review board of Tokyo Medical University (No. 3031).
We diagnosed the patients as having definitive Meniere’s disease, according to the 1995 Guidelines of the Committee of Hearing and Equilibrium of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS)7), with slight modification. In Japan, routine hearing tests are usually performed beyond the 3-kHz hearing level. Thus, for this study we tested all subjects at four frequencies (0.5, 1, 2, and 4 kHz), recording the worst pure-tone audiometry (PTA) results, instead of the results at the four frequencies (0.5, 1, 2, 3 kHz) recommended by the AAO-HNS 1995 guidelines7). The patients were divided into four groups based on their disease classification in relation to the four stages described in the AAO-HNS guidelines. All patients underwent nystagmic examinations and audiometric measurements, including hearing testing (PTA) and tympanometry.
Subjects used a MC-510 clinical thermometer (OMRON, Japan) to measure their TMT bilaterally. All thermometers were precisely calibrated by the manufacturer prior to shipping to our clinic. MC-510 clinical thermometers detect infrared heat given off by the eardrum and surrounding tissues.
The MC-510 thermometer has two measurement modes:a fast one-second mode and a unique directional mode. In the fast one-second mode, the probe is inserted into the ear as far as possible in the direction of the eardrum and the “on” switch is pressed while the unit is in the ear. If the probe is in the appropriate position, measurement finishes in approximately one second. When a clinician or patient is experienced in taking temperatures from the ear, therefore, this mode is especially effective. In unique directional mode, the probe is inserted into the ear and shifted slightly to the left and right. Within no more than 10 seconds, the thermometer automatically searches for the correct direction toward the ear drum that emits infrared heat. Measurement time is longer than in the fast one-second mode, but this mode is not affected by the measurer’s experience, and measurement error is very minimal23). Therefore, when a patient or family member is somewhat unfamiliar with taking temperature from the ear, this mode is probably more suitable. For this reason, we chose to use the unique directional mode as the measurement method of choice in the present study. In order to improve the accuracy of temperature measurements, only one clinical staff member experienced in taking temperature from the ear was permitted to teach all patients on how to take their temperature using this measurement method. Each patient was given a MC-510 clinical thermometer to monitor and record their temperature. Moreover, to investigate the effect of environmental temperature and thermometer precision on TMT, we conducted two feasibility studies simultaneously in 264 volunteers (84 male and 180 females; age:16-84 years;mean±SD:51±17 years). All 264 volunteers had no detectable middle ear disease. In study 1, after resting for more than 10 minutes, subjects measured their own TMT with the same thermometer used by the clinical staff member. The measurements were carried out twice on the same ear at intervals of >1 minute, and the measurements were compared. In study 2, we examined the correlation between the first TMT value measured in study 1 and the environmental temperature.
Subjects were instructed to measure their TMT (both left and right ear) at 7:00 a.m. each day for a period of 2 to 4 weeks. To avoid any potential effects of body and head position on TMT19), we instructed all subjects to measure bilateral TMT in the same sitting position every time. They were also instructed to note the presence of vertigo attacks and their physical condition. After collecting the subjects’ TMT measurements and notes, we then researched and tabulated Tokyo weather data (e.g., mean atmospheric pressure, mean atmospheric temperature, and mean humidity values) from the Japan Meteorological Agency (JMA) for the period when subjects measured their TMTs. We included the weather data in our records. Generally, TMT tends to be affected by environmental temperature12,23). Therefore, we decided to use the ratio of TMT measured in the affected ear (Ta) to that in the unaffected ear (Tu) for our analyses. We refer to this ratio as percent TMT asymmetry (%TMTA), and we calculated it according to the following equation:100(Ta−Tu)/(Ta+Tu). In healthy volunteers, we operationally defined %TMTA as follows:100(Tr−Tl)/(Tr+Tl) or 100(Tl−Tr)/(Tl+Tr), where Tr represents TMT in the right ear, and Tl represents TMT in the left ear.
We compared the results obtained from the healthy volunteers with those obtained from the subjects with Meniere’s disease. For statistical analyses, we used the T test and Pearson contingency coefficients to assess %TMTA across patients. P values of <0.05 were considered significant. Calculations were performed with Stat Mate 3 software (Atoms, Japan).
III. RESULTS
1. Thermometer precision assessment
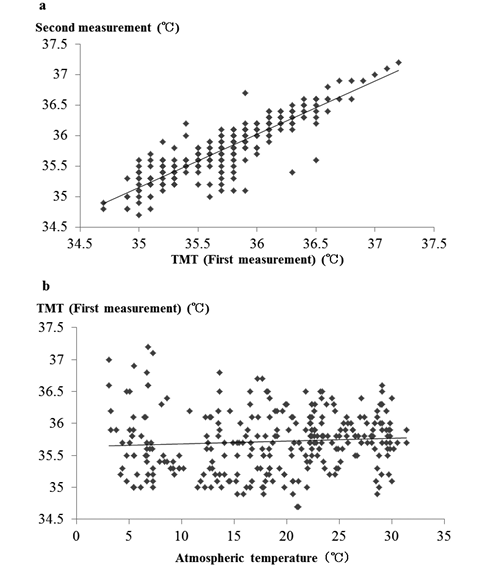
In 264 volunteers, the mean TMT±SD was 35.72±0.47° (first measurement) and 35.78±0.47° (second measurement);these two measurements did not differ significantly (Fig. 1a). The mean difference between the two TMT measurements was 0.06±0.24°. Environmental temperature did not significantly affect the first TMT measurement (Fig. 1b). Moreover, environmental temperature did not affect mean difference in TMT values.
 |
|
Fig. 1. TMT results in 264 volunteers (feasibility studies).
a. Comparison of first and second TMT measurements.
In order to verify the precision of the thermometer we used, healthy volunteers were asked to measure their TMT twice. We then compared the two measurements. There was no significant difference between the first and second TMT measurements. TMT:tympanic membrane temperature.
b. Variation of TMT with environmental temperature.
We also determined whether environmental temperature influenced the TMT of healthy volunteers. Environmental temperature did not significantly affect TMT values. |
2. Healthy volunteers and patients with Meniere’s disease
Table 1 summarizes characteristics of the healthy volunteers. In healthy volunteers, the mean %TMTA±SD was −0.07±0.28 (right-left) or 0.07±0.28 (left-right), and the mean±SD TMT measurement period was 29±1 days (Table 1). Their past alcohol drinking and smoking history and past medical history did not affect %TMTA.
Table 2 shows the results of all patients with Meniere’s disease. There were no significant age differences between Meniere’s disease patients and healthy volunteers. The mean %TMTA±SD of the Meniere’s disease patients was 0.11±0.59, and their mean measurement period±SD was 24±6 days. The mean length±SD of vertigo attacks was 4±3 days. All of the patients’ tympanometry results were normal (Jerger A type). The patients were classified into four groups based on the four stages of Meniere’s disease severity, following the AAO-HNS classification scheme:stage 1 (n=8);stage 2 (n=2);stage 3 (n=1);and stage 4 (n=0). There was no significant difference between the %TMTA of the four groups. Furthermore, %TMTA did not significantly correlate with PTA, stage, or vertigo recurrence rate. As with the health volunteers, the drinking and smoking history and past medical history of the Meniere’s disease patients did not affect %TMTA.
Table 3 presents a summary of the mean %TMTA values of healthy volunteers and Meniere’s disease patients. In the Meniere’s disease patients, the mean %TMTA±SD measured during the paroxysmal period (with vertigo) was 0.19±0.62;whereas the mean %TMTA±SD measured during the interictal period (without vertigo) was 0.10±0.58. Although the mean %TMTA±SD values did not significantly differ between the two groups, the values measured during the paroxysmal period tended to be higher to some extent. The overall mean %TMTA of Meniere’s disease patients was not significantly different compared to that of healthy volunteers. However, the mean %TMTA of the patients was significantly greater than that of healthy volunteers during the paroxysmal period (p<0.001, p<0.05).
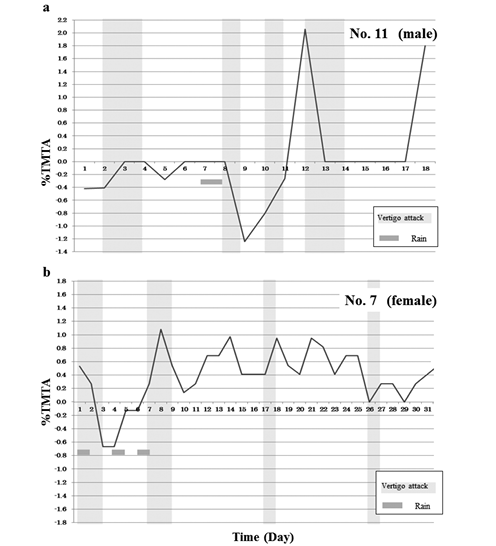
Examples of %TMTA fluctuations observed in patients (Nos. 7 and 11) with Meniere’s disease are shown in Fig. 2a and 2b. %TMTA in patients tended to be positive. This was especially the case in patients experiencing vertigo attacks, where %TMTA increased, spiking toward positive values.
The relationship between patients’ vertigo attacks and the weather data is presented in Table 4. Of the various meteorological parameters, only humidity values during the paroxysmal period were significantly greater than those during the interictal period. However, regardless of whether a subject experienced vertigo or not, %TMTA values did not significantly correlate with atmospheric pressure, temperature, or humidity.
| Table 1. Characteristics and results of healthy volunteers |
| No |
Age |
Sex |
Drinking history |
Smokinghistory |
Past medical historyexcept for inner ear dysfunction |
History of inner ear dysfunction |
%TMTA
(right-left) |
Measurementperiod (days) |
|
| 1 |
36 |
F |
Twice a week |
No |
hypothyroidism |
no |
−0.06±0.15 |
30 |
| 2 |
40 |
M |
Twice a week |
No |
no |
no |
−0.01±0.19 |
31 |
| 3 |
49 |
F |
Twice a week |
No |
no |
no |
−0.09±0.56 |
30 |
| 4 |
49 |
F |
No |
No |
hypertension |
no |
0.01±0.35 |
30 |
| 5 |
57 |
F |
Twice a month |
No |
hypertension |
no |
−0.17±0.11 |
27 |
| 6 |
59 |
F |
Twice a week |
No |
no |
no |
0.00±0.11 |
30 |
| 7 |
59 |
F |
Twice a week |
No |
osteoporosis |
no |
−0.11±0.31 |
29 |
| 8 |
70 |
F |
No |
No |
no |
no |
−0.13±0.14 |
28 |
| %TMTA:percent tympanic membrane temperature. |
| Table 2. Patients with Meniere’s disease:characteristics, health behaviors, and medical history, hearing test results, and frequency of vertigo attacks |
| No |
Age |
Sex |
Drinkinghistory |
Smokinghistory |
Past medical history |
Pure tone audiometry |
Tympanometry |
%TMTA |
Measurement period |
| (dB) |
Stage |
Period (days) |
Vertigo attack |
| day |
% |
|
| 1 |
40 |
F |
n/a |
n/a |
hyperthyroidism |
6.3 |
1 |
A |
0.06±0.33 |
20 |
8 |
40 |
| 2 |
38 |
M |
n/a |
n/a |
no |
7.5 |
1 |
A |
0.62±0.70 |
25 |
8 |
32 |
| 3 |
65 |
F |
n/a |
n/a |
no |
12.5 |
1 |
A |
−0.12±0.26 |
28 |
2 |
7 |
| 4 |
83 |
F |
No |
No |
hypertension/hyperlipemia |
12.5 |
1 |
A |
0.17±0.29 |
18 |
4 |
22 |
| 5 |
73 |
F |
n/a |
n/a |
hypertension |
16.3 |
1 |
A |
−0.66±0.85 |
26 |
3 |
12 |
| 6 |
35 |
F |
n/a |
n/a |
no |
17.5 |
1 |
A |
0.35±0.47 |
14 |
2 |
14 |
| 7 |
54 |
F |
n/a |
n/a |
no |
21.3 |
1 |
A |
0.39±0.41 |
31 |
6 |
19 |
| 8 |
66 |
M |
Twice a week |
No |
no |
21.3 |
1 |
A |
0.12±0.15 |
27 |
1 |
4 |
| 9 |
67 |
F |
once a week |
No |
no |
35.0 |
2 |
A |
0.05±0.15 |
23 |
6 |
26 |
| 10 |
61 |
F |
n/a |
n/a |
no |
40.0 |
2 |
A |
0.30±0.34 |
30 |
1 |
3 |
| 11 |
71 |
M |
n/a |
n/a |
no |
67.5 |
3 |
A |
0.14±1.08 |
18 |
6 |
33 |
| *Meniere’s disease stages were based on the extent of hearing loss, as described by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS):Stage 1, ≦25 dB;Stage 2, 26-40 dB;Stage 3, 41-70 dB;Stage 4, >70 dB. %TMTA:Percent tympanic membrane temperature asymmetry;n/a:not available. |
| Table 3. Summary of mean %TMTA values in healthy volunteers and Meniere’s disease patients |
|
Total |
|
with vertigo |
without vertogo |
|
| Control |
−0.07±0.28*†‡ |
(right-left) |
|
|
| 0.07±0.28** |
(left-right) |
|
|
|
| MD |
0.11±0.59‡ |
|
0.19±0.62*,** |
0.10±0.58† |
There were significant differences between the same symbols (*, **, †and ‡).
*†‡:p<0.001, **:p<0.05.
MD:Meniere’s disease;%TMTA:percent tympanic membrane temperature. |
 |
|
Fig. 2. Example of fluctuations in %TMTA in a patient with Meniere’s disease.
%TMTA here was affected by weather in this patient.
%TMTA values spiked upward during vertigo attacks (light gray vertical bars). Gray squares mark individual rainy days.
%TMTA, Percent tympanic membrane temperature asymmetry. |
IV. DISCUSSION
Before we conducted the case study, we determined the precision of the thermometers that would be used for TMT measurements. To confirm the precision of the thermometer, we investigated variations in TMT continuously and made two TMT measurements. We also examined whether environmental temperature affected the TMT measurements. There was no significant difference between the two TMT measurements. Moreover, environmental temperature did not affect the TMT values (Fig. 1a, b). Kishi et al.12) reported that using an older version (MC-500) of the model than the one (MC-510) we used in the present study, TMT varied over time with environmental temperature, consumption of saccharide, extent of exercise, and chewing gum usage. They also observed that TMT was barely affected by environmental temperature if they waited a sufficient amount of time before taking another measurement, which was consistent with our observations.
| Table 4. Relationship between Meniere’s disease patients’ vertigo attacks and weather(atmospheric pressure, temperature, and humidity) |
|
%TMTA |
Atmospheric pressure(hPa) |
Atmospheric temperature(degrees) |
Humidity(percent) |
|
| with vertigo |
0.19±0.62 |
1,010.3±5.4 |
13.0±7.7 |
61.2±14.9* |
|
| without vertigo |
0.10±0.58 |
1,011.6±6.2 |
11.8±7.2 |
55.2±16.7* |
*:p<0.05.
%TMTA:percent tympanic membrane temperature. |
The vestibular end organs are supplied by two arteries, the anterior and posterior vestibular artery. The former is a direct branch of the anterior inferior cerebellar artery and supplies the utricle and the superior and horizontal canal ampullae. The latter is a branch of the common cochlear artery and supplies the saccule and posterior canal ampulla. However, these two arteries contribute very little to the arterial blood going to the endolymphatic sac13). The arterial supply to the endolymphatic sac comes from two different sources:the posterior vestibular artery27) and the posterior meningeal artery, a branch of the external carotid artery that supplies the bulk of arterial blood to the endolymphatic sac2,22).
Autonomic dysfunction has been proposed to be one cause of Meniere’s disease8,21,32). Several studies have suggested that in vertigo, asymmetry in the organization of the autonomic nervous system causes asymmetrical excitability of the vestibular system, resulting in nystagmus and vertigo20,29). The same studies found a correlation between this asymmetry and side differences in vertebral blood flow. In Meniere’s disease, asymmetrical vertebral blood flow induced by asymmetrical sympathetic activity causes inner ear ischemia, resulting in hydrops16). This is consistent with the finding that the endolymphatic sac is innervated by sympathetic fibers, which could control local blood circulation14).
Bezinger believed that regional heat flow is the strongest factor influencing TMT4). Decreased cerebral blood flow induces heat production in cerebral tissues, resulting in a rise in TMT18). This is why TMT is a useful indicator of brain temperature4,18). The tympanic membrane is supplied mainly by the maxillary artery, a branch of the external carotid, and by one branch of the internal carotid artery6). These previous findings led us to hypothesize that TMT might be useful for estimating blood flow to the endolymphatic sac, which is supplied by the external carotid artery. Thus, an increase or decrease in %TMTA could indirectly reflect perfusion and be a relative indicator of ischemia or reperfusion, respectively, of the endolymphatic sac in the affected side.
These results also suggest a close correlation between %TMTA and endolymphatic hydrops. Lee and Kimura reported that interruption of the blood supply to the endolymphatic sac results in atrophy of the sac epithelium, loss of intraluminal precipitates, and development of labyrinthine hydrops14). Ikeda and Sando also reported that in Meniere’s disease, a decrease in vascular density occurs in the endolymphatic sac, a potential factor in the pathogenesis of endolymphatic hydrops11). Their findings indicate that ischemia in the endolymphatic sac leads to hydrops, which supports our hypothesis. Moreover, ischemia in the endolymphatic sac might also lead to a sudden fluctuation in %TMTA during the paroxysmal period (Fig. 2a, b).
Several studies have shown that hydroxyl radicals play an important role in cochlear ischemia-reperfusion injuries9,28). The mechanism underlying cochlear injury in individuals with endolymphatic hydrops and secondary dysfunction appears to be neurotoxicity related5). Several studies proposed that oxidative insult is likely to contribute to the pathology associated with endolymphatic hydrops10,26,30). Indeed, free radical scavengers have proven to be useful for treating endolymphatic hydrops10,26,30). Future studies, therefore, should examine the relationship between free radical scavenger treatments and TMT changes.
Several studies have shown a close relationship between vertigo attacks and weather17,24). According to Salt, over 70% of patients with Meniere’s disease prone to vertigo attacks note symptoms that are brought on by weather changes24). Mizukoshi et al. reported that passing cold fronts influence the onset of Meniere’s disease17). Yasuda et al. also noted an obvious relationship between the time of occurrence of attacks and atmospheric pressure changes and rapid falling temperatures33). Alternobaric vertigo (AV) is a well-known type of vertigo that occurs as a result of atmospheric pressure changes15). Surprisingly in our study, atmospheric pressure and temperature did not affect vertigo attacks. Besides, humidity values during the paroxysmal period were significantly greater than those during the interictal period. Our extensive review of the literature failed to identify any study on the relationship between humidity and vertigo. Therefore, it is not clear how humidity affects vertigo attacks directly. High humidity caused our Meniere’s disease patients much discomfort. As a result, this could lead to secondary vertigo attacks, as is seen with changes in atmospheric pressure and temperature17,30,33). Therefore, we hypothesized that, like atmospheric pressure and temperature, humidity also could be an important climatic factor in vertigo attacks. In the present study, there was not a significant difference between %TMTA and weather data, indicating that weather changes did not affect %TMTA. However, we observed that the %TMTA in several subjects sometimes increased after a rainy day (Fig. 2a, b). These results suggest that the vestibular stimulation caused by the change in weather might produce a decrease in cerebral blood flow1), which could lead to secondary ischemia/reperfusion in the endolymphatic sac. This provides a secondary explanation for why the %TMTA of certain subjects increases after a rainy day.
V. CONCLUSIONS
In summary, our study provides evidence to support the practice of measuring TMT to evaluate endolymphatic hydrops. Measuring TMT is easy and non-invasive, so it is useful for self-management in patients. Further investigations are needed to identify additional factors that influence TMT. One clear next step would be to evaluate TMT in vertigo patients who do not have endolymphatic hydrops, such as patients with vestibular neuritis, benign paroxysmal positional vertigo, among others.
REFERENCES
1) Adachi, T., Sato, A., Pauzi, A. and Yusof, M.:Effect of vestibular stimulation on cerebral blood flow in anesthetized rat. Otologia (Fukuoka), 37, 1224-1229, 1991.
2) Bast, T.H. and Anson, B.J.:The temporal bone and the ear. Charles C Thomas, Springfield, Illinois, pp. 257-258, 1949.
3) Bezinger, T.H.:On physical heat regulation and the sense of temperature in man. Proc. Natl. Acad. Sci. USA., 45, 645-659, 1959.
4) Bezinger, T.H.:Clinical temperature. JAMA, 209(8), 1200-1206, 1969.
5) Bixenstine, P.J., Maniglia, M.P., Vasanji, A., Alagraman, K.N. and Megerian, C.A.:Spiral ganglion degeneration patterns in endolymphatic hydrops. Laryngoscope, 118, 1217-1223, 2008.
6) Brinnel, H. and Michel, C.:Tympanic temperature is a core temperature in humans. J. Therm. Biol., 14, 47-53, 1989.
7) Committee on Hearing and Equilibrium.:Guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. Otolaryngol. Head Neck Surg., 113, 181-185, 1995.
8) Eccles, R. and Eccles, K.S.:Asymmetry in the autonomic nervous system with reference to the nasal cycle, migraine, anisocoria and Menière’s syndrome. Rhinology, 19, 121-125, 1981.
9) Hakuba, N., Matsubara, A., Hyodo, J., Taniguchi, M., Maetani, T., Shimizu, Y., Tsujiuchi, Y., Shudou, M. and Gyo, K.:AMPA/kainite-type glutamate receptor antagonist reduces progressive inner hair cell loss after transient cochlear ischemia. Brain Res., 979, 194-202, 2003.
10) Horner, K.C. and Guilhaume, A.:Ultrastructural changes in the hydropic cochlea of the guinea pig. Eur. J. Neurosci., 7, 1305-1312, 1995.
11) Ikeda, M. and Sando, I.:Histopathological studies in Meniere’s disease. Vascularity of the endolymphatic sac. Ann. Otol. Rhinol. Laryngol., 94(Suppl. 118), 6-10, 1985.
12) Kishi, T., Matsumoto, T. and Ikeda, S.:Evaluation of tympanic membrane temperature with non-contact ear type clinical thermometer. Nihon Univ. J. Oral Sci., 24, 275-280, 1998.
13) Kimura, R.S. and Perlman, H.B.:Arterial obstruction of the labyrinth. Part II. Vestibular changes. Ann. Otol. Rhinol. Laryngol., 67, 26-41, 1958.
14) Lee, K.S. and Kimura, R.S.:Ischemia of the endolymphatic sac. Acta Otolaryngol. (stockh), 112, 658-666, 1992.
15) Lundgren, C.E.G.:Alternobaric vertigo-a diving hazard. Br. J. Med., 2, 511-512, 1965.
16) Matsunaga, T., Yamamoto, K., Kubo, T., Ogino, H., Ochiai, K. and Takeda, N.:Sympatho-vascular mechanism of vertigo attack in Meniere’s disease. In:Meniere’s Disease: Perspectives in ’90s, Eds. by R. Filipo and M. Barbara. Kugler, Amsterdam/ New York, pp. 77-80, 1994.
17) Mizukoshi, K., Watanabe, Y., Shojaku, H., Ito, M., Ishikawa, M., Aso, S., Asai, M. and Motoshima, H.:Influence of cold front upon the onset of Meniere’s disease in Toyama. Acta Otolaryngol. (Stockh), Suppl., 520, 412-414, 1995.
18) Moriya, K., Sekitani, T., Yamashita, H. and Mizokami, H.: Tympanic membrane temperature in a patient with vertigo. Acta Otolaryngol. (Stockh), Suppl., 506, 24-25, 1993.
19) Ogawa, T., Sugenoya, J., Ohnishi, N., Natsume, K., Imai, K., Kandori, Y., Ishizuka, A. and Osada, A.:Effects of body and head positions on bilateral difference in tympanic temperatures. Eur. J. Appl. Physiol., 67, 354-359, 1993.
20) Ogino, H., Tanaka, M. and Matsunaga, T.:Autonomic nervous function in vertiginous patients?side difference in Meniere’s disease?. Pract. Otol. (Kyoto), Suppl. 8, 191-200, 1986.
21) Pappas, D.G., Crawford, W. and Coghlan, H.C.:Dizziness and the autonomic dysfunction syndrome. Otolaryngol. Head Neck Surg., 94, 186-194, 1986.
22) Rask-Andersen, H.:The vascular supply of the endolymphatic sac. Acta Otolaryngol. (Stockh), 88, 315-327, 1979.
23) Saito, N.:Evaluating the performance of the aural thermometer. The Journal of Ambulatory and General Pediatrics, 4, 324-327, 2001.
24) Salt, A.:Survey of the effects of pressure on Meniere’s disease. http://oto.wustl.edu/men/pressure/2000.
25) Sekitani, T., Mizokami, H., Imate, Y., Yamashita, H., Moriya, K., Hasuike, K., Ikeda, T. and Hara, J.:Temperature of tympanic membrane of the vertiginuous patient. Equilibrium Res., Suppl. 9, 39-45, 1993.
26) Shinomori, Y. and Kimura, R.S.:Allopurinol attenuates endolymphatic hydrops in the guinea pig cochlea. ORL J. Otorhinolaryngol. Relat. Spec., 63, 267-271, 2001.
27) Smith, C.A.:The capillaries of the vestibular membranous labyrinth in the guinea pig. Laryngoscope, 63, 87-104, 1953.
28) Tabuchi, K., Tsuji, S., Fujihira, K., Oikawa, K., Hara, A. and Kusakari, J.:Outer hair cells functionally and structurally deteriorate during reperfusion. Hear Res., 173, 153-163, 2002.
29) Takeda, N., Taya, N., Nakagami, T., Koizuka, I., Kawasaki, Y., Morita, M., Ogino, H., Yamatodani, A., Kubo, T. and Matsunaga, T.:Orthostatic hypotension with vertigo: Relationship between asymmetrical vertebral blood flow and nystagmus. Equilibrium Res., 55, 364-370, 1966.
30) Takumida, M., Takeda, T., Takeda, S., Kakigi, A., Nakatani, H. and Anniko, M.:Protective effect of edaravone against endolymphatic hydrops. Acta Otolaryngol., 127, 1124-1131, 2007.
31) Watanabe, Y., Shojaku, H., Ito, M., Ishikawa, M. and Mizukoshi, K.:Influence of a cold front upon the onset of Meniere’s disease. Equilibrium Res., suppl., 9, 21-24, 1993.
32) Williams Jr, H.L.:A review of the literature as to the physiologic dysfunction of Meniere’s disease:a new hypothesis as to its fundamental cause. Laryngoscope, 75, 1661-1689, 1965.
33) Yasuda, H., Tokita, T., Miyata, H., Takayasu, S., Katori, S., Yamazaki, S., Sakai, N., Yamakawa, M. and Yanai, O.:An epidemiological study of attack of Meniere’s disease and its relationship with meteorological phenomena in four isolated regions of Japan. Otologia (Fukuoka), 24, 946-954, 1978.
連絡先 〒179-0073 東京都練馬区田柄1-15-15
北島耳鼻咽喉科医院
北島 尚治
TEL:03(3930)4830,FAX:03(3976)0873
E-mail:nao-ake@bk2.so-net.ne.jp

