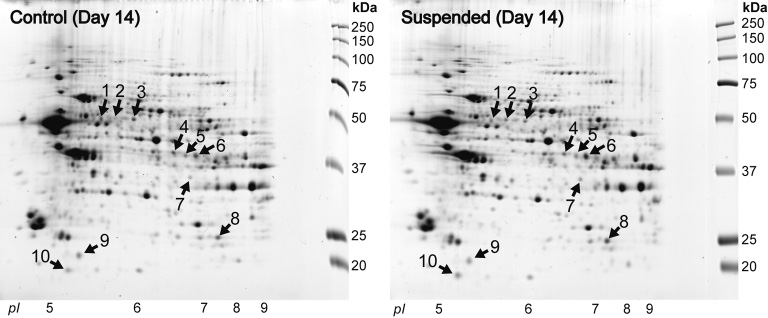
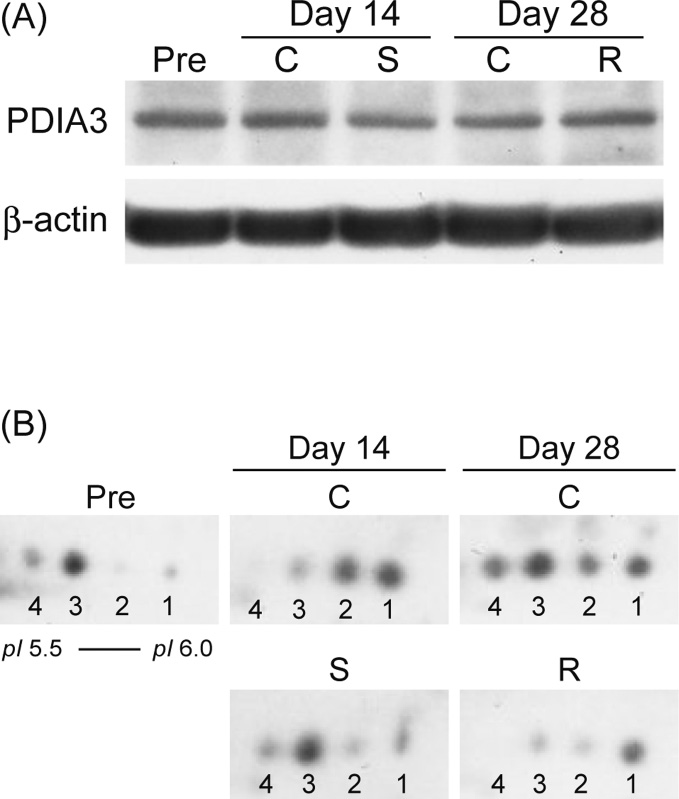
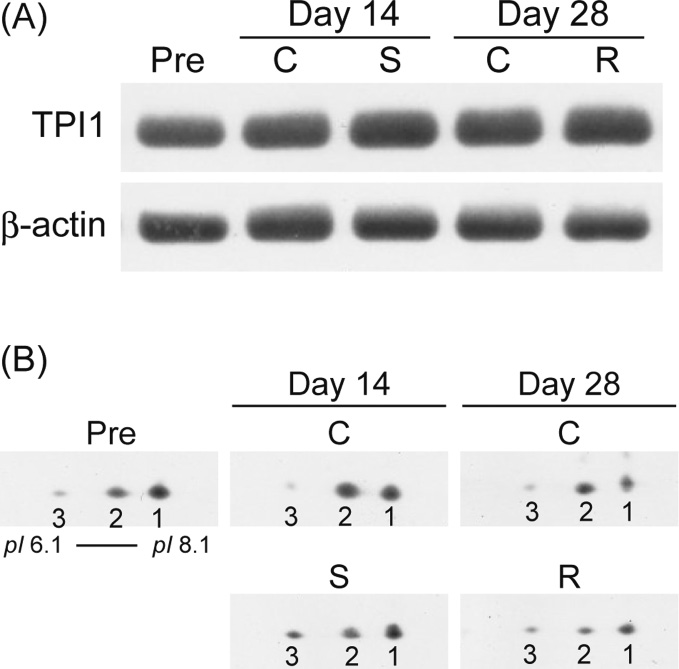
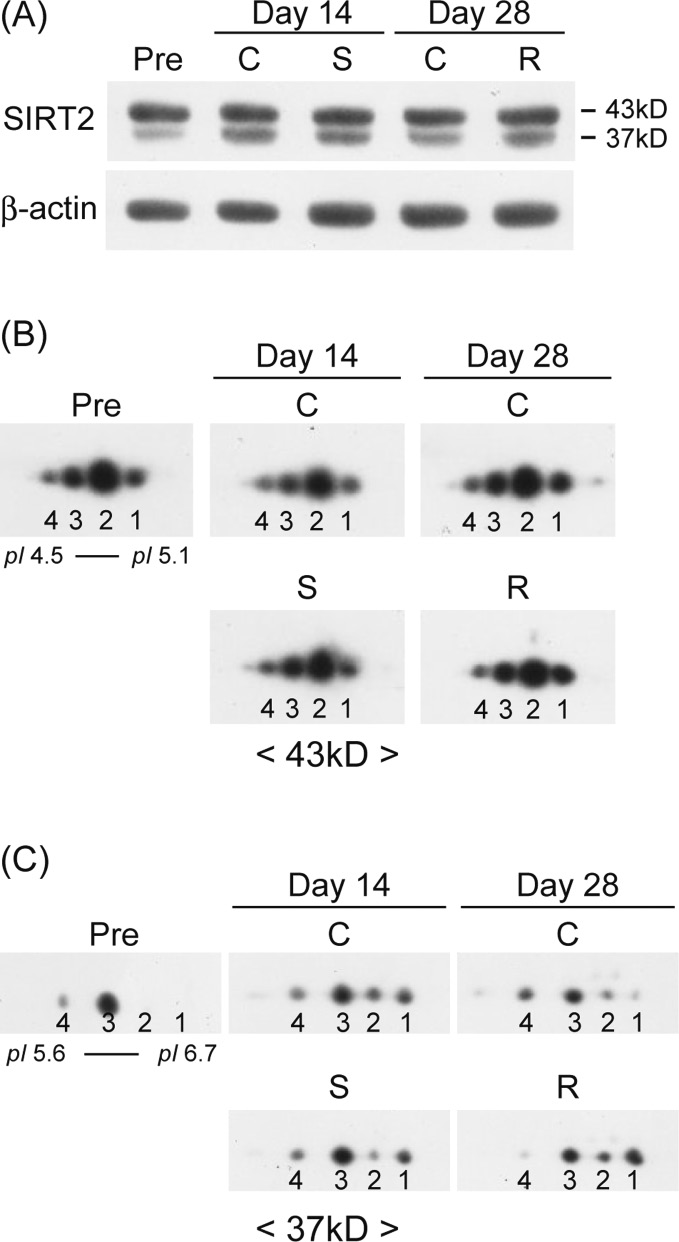
Modulation of Hippocampal Proteins by Exposure to Simulated Microgravity Environment during the Postnatal Development in Rats
| 1) |
Bak, L.K., Schousboe, A. and Waagepetersen, H.S. : The glutamate/GABA-glutamine cycle : aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem., 98, 641-653, 2006. |
| 2) |
Boulos, S., Meloni, B.P., Arthur, P.G., Bojarski, C. and Knuckey, N.W. : Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J. Neurosci. Res., 85, 3089-3097, 2007. |
| 3) |
Calas, A.G., Richard, O., Meme, S., Beloeil, J.C., Doan, B.T., Gefflaut, T., Meme, W., Crusio, W.E., Pichon, J. and Montecot, C. : Chronic exposure to glufosinate-ammonium induces spatial memory impairments, hippocampal MRI modifications and glutamine synthetase activation in mice. Neurotoxicology, 29, 740-747, 2008. |
| 4) |
Cole, A.R., Knebel, A., Morrice, N.A., Robertson, L.A., Irving, A.J., Connolly, C.N. and Sutherland, C. : GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J. Biol. Chem., 279, 50176-50180, 2004. |
| 5) |
D’Amelio, F., Fox, R.A., Wu, L.C. and Daunton, N.G. : Quantitative changes of GABA-immunoreactive cells in the hindlimb representation of the rat somatosensory cortex after 14-day hindlimb unloading by tail suspension. J. Neurosci. Res., 44, 532-539, 1996. |
| 6) |
DeFelipe, J., Arellano, J.I., Merchan-Perez, A., Gonzalez-Albo, M.C., Walton, K. and Llinas, R. : Spaceflight induces changes in the synaptic circuitry of the postnatal developing neocortex. Cereb. Cortex, 12, 883-891, 2002. |
| 7) |
De Santo, N.G., Christensen, N.J., Drummer, C., Kramer, H.J., Regnard, J., Heer, M., Cirillo, M. and Norsk, P. : Fluid balance and kidney function in space : introduction. Am. J. Kidney Dis., 38, 664-667, 2001. |
| 8) |
Edgerton, V.R., McCall, G.E., Hodgson, J.A., Gotto, J., Goulet, C., Fleischmann, K. and Roy, R.R. : Sensorimotor adaptations to microgravity in humans. J. Exp. Biol., 204, 3217-3224, 2001. |
| 9) |
Feldmann, R.E. Jr., Maurer, M.H., Hunzinger, C., Lewicka, S., Buergers, H.F., Kalenka, A., Hinkelbein, J., Broemme, J.O., Seidler, G.H., Martin, E. and Plaschke, K. : Reduction in rat phosphatidylethanolamine binding protein-1 (PEBP1) after chronic corticosterone treatment may be paralleled by cognitive impairment : a first study. Stress, 11, 134-147, 2008. |
| 10) |
Ford, J.H. : Spindle microtubular dysfunction in mothers of Down syndrome children. Hum. Genet., 68, 295-298, 1984. |
| 11) |
Frigeri, A., Iacobas, D.A., Iacobas, S., Nicchia, G.P., Desaphy, J.F., Camerino, D.C., Svelto, M. and Spray, D.C. : Effect of microgravity on gene expression in mouse brain. Exp. Brain Res., 191, 289-300, 2008. |
| 12) |
George, A.J., Holsinger, R.M., McLean, C.A., Tan, S.S., Scott, H.S., Cardamone, T., Cappai, R., Masters, C.L. and Li, Q.X. : Decreased phosphatidylethanolamine binding protein expression correlates with Abeta accumulation in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol. Aging, 27, 614-623, 2006. |
| 13) |
Guix, F.X., Ill-Raga, G., Bravo, R., Nakaya, T., de Fabritiis, G., Coma, M., Miscione, G.P., Villa-Freixa, J., Suzuki, T., Fernandez-Busquets, X., Valverde, M.A., de Strooper, B. and Munoz, F.J. : Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain, 132, 1335-1345, 2009. |
| 14) |
Gulesserian, T., Kim, S.H., Fountoulakis, M. and Lubec, G. : Aberrant expression of centractin and capping proteins, integral constituents of the dynactin complex, in fetal down syndrome brain. Biochem. Biophys. Res. Commun., 291, 62-67, 2002. |
| 15) |
Harting, K. and Knoll, B. : SIRT2-mediated protein deacetylation : An emerging key regulator in brain physiology and pathology. Eur. J. Cell. Biol., 89, 262-269, 2010. |
| 16) |
Hebert, D.N. and Molinari, M. : In and out of the ER : protein folding, quality control, degradation, and related human diseases. Physiol. Rev., 87, 1377-1408, 2007. |
| 17) |
Hetz, C., Russelakis-Carneiro, M., Walchli, S., Carboni, S., Vial-Knecht, E., Maundrell, K., Castilla, J. and Soto, C. : The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J. Neurosci., 25, 2793-2802, 2005. |
| 18) |
Inagaki, N., Chihara, K., Arimura, N., Menager, C., Kawano, Y., Matsuo, N., Nishimura, T., Amano, M. and Kaibuchi, K. : CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci., 4, 781-782, 2001. |
| 19) |
Ishihara, A., Ohira, Y., Roy, R.R., Nagaoka, S., Sekiguchi, C., Hinds, W.E. and Edgerton, V.R. : Effects of 14 days of spaceflight and nine days of recovery on cell body size and succinate dehydrogenase activity of rat dorsal root ganglion neurons. Neuroscience, 81, 275-279, 1997. |
| 20) |
Johnston-Wilson, N.L., Sims, C.D., Hofmann, J.P., Anderson, L., Shore, A.D., Torrey, E.F. and Yolken, RH. : Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol. Psychiatry, 5, 142-149, 2000. |
| 21) |
Kawano, F., Goto, K., Wang, X.D., Terada, M., Ohira, T., Nakai, N., Yoshioka, T. and Ohira, Y. : Role(s) of gravitational loading during developing period on the growth of rat soleus muscle fibers. J. Appl. Physiol., 108, 676-685, 2010. |
| 22) |
Kawano, F., Ishihara, A., Stevens, J.L., Wang, X.D., Ohshima, S., Horisaka, M., Maeda, Y., Nonaka, I. and Ohira, Y. : Tension- and afferent input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am. J. Physiol. Regul. Integr. Comp. Physiol., 287, R76-86, 2004. |
| 23) |
Kawano, F., Matsuoka, Y., Oke, Y., Higo, Y., Terada, M., Wang, X.D., Nakai, N., Fukuda, H., Imajoh-Ohmi, S. and Ohira, Y. : Role(s) of nucleoli and phosphorylation of ribosomal protein S6 and/or HSP27 in the regulation of muscle mass. Am. J. Physiol. Cell Physiol., 293, C35-44, 2007. |
| 24) |
Krapfenbauer, K., Engidawork, E., Cairns, N., Fountoulakis, M. and Lubec, G. : Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res., 967, 152-160, 2003. |
| 25) |
Lee, W.H., Choi, J.S., Byun, M.R., Koo, K.T., Shin, S., Lee, S.K. and Surh, Y.J. : Functional inactivation of triosephosphate isomerase through phosphorylation during etoposide-induced apoptosis in HeLa cells : Potential role of Cdk2. Toxicology, 278(2), 224-228, 2010. |
| 26) |
Maki, M., Matsukawa, N., Yuasa, H., Otsuka, Y., Yamamoto, T., Akatsu, H., Okamoto, T., Ueda, R. and Ojika, K. : Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer disease. J. Neuropathol. Exp. Neurol., 61, 176-185, 2002. |
| 27) |
Nomura, S., Kami, K., Kawano, F., Oke, Y., Nakai, N., Ohira, T., Fujita, R., Terada, M., Imaizumi, K. and Ohira, Y. : Effects of hindlimb unloading on neurogenesis in the hippocampus of newly weaned rats. Neurosci. Lett., in press. |
| 28) |
Ohira, Y., Nomura, T., Kawano, F., Sato, Y., Ishihara, A. and Nonaka, I. : Effects of nine weeks of unloading on neuromuscular activities in adult rats. J. Gravit. Physiol., 9, 49-59, 2002. |
| 29) |
Ohira, Y., Yoshinaga, T., Ohara, M., Nonaka, I., Yoshioka, T., Yamashita-Goto, K., Shenkman, B.S., Kozlovskaya, I.B., Roy, R.R. and Edgerton, V.R. : Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol., 87, 1776-1785, 1999. |
| 30) |
Ojika, K., Mitake, S., Kamiya, T., Kosuge, N. and Taiji, M. : Two different molecules, NGF and free-HCNP, stimulate cholinergic activity in septal nuclei in vitro in a different manner. Brain Res. Dev. Brain Res., 79, 1-9, 1994. |
| 31) |
Pandithage, R., Lilischkis, R., Harting, K., Wolf, A., Jedamzik, B., Luscher-Firzlaff, J., Vervoorts, J., Lasonder, E., Kremmer, E., Knoll, B. and Luscher, B. : The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J. Cell Biol., 180, 915-929, 2008. |
| 32) |
Petralia, R.S., Sans, N., Wang, Y.X. and Wenthold, R.J. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol. Cell Neurosci., 29, 436-452, 2005. |
| 33) |
Reynolds, A.J., Bartlett, S.E. and Hendry, I.A. : Molecular mechanisms regulating the retrograde axonal transport of neurotrophins. Brain Res. Brain Res. Rev., 33, 169-178, 2000. |
| 34) |
Sarkar, P., Sarkar, S., Ramesh, V., Hayes, B.E., Thomas, R.L., Wilson, B.L., Kim, H., Barnes, S., Kulkarni, A., Pellis, N. and Ramesh, G.T. : Proteomic analysis of mice hippocampus in simulated microgravity environment. J. Proteome Res., 5, 548-553, 2006. |
| 35) |
Sarkar, P., Sarkar, S., Ramesh, V., Kim, H., Barnes, S., Kulkarni, A., Hall, J.C., Wilson, B.L., Thomas, R.L., Pellis, N.R. and Ramesh, G.T. :Proteomic analysis of mouse hypothalamus under simulated microgravity. Neurochem. Res., 33, 2335-2341, 2008. |
| 36) |
Schneider, A.S. : Triosephosphate isomerase deficiency : historical perspectives and molecular aspects. Baillieres Best Pract. Res. Clin. Haematol., 13, 119-140, 2000. |
| 37) |
Simzar, S., Ellyin, R., Shau, H. and Sarafian, T.A. : Contrasting antioxidant and cytotoxic effects of peroxiredoxin I and II in PC12 and NIH3T3 cells. Neurochem. Res., 25, 1613-1621, 2000. |
| 38) |
Wang, X.D., Kawano, F., Matsuoka, Y., Fukunaga, K., Terada, M., Sudoh, M., Ishihara, A. and Ohira, Y. : Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol., 290, C981-989, 2006. |
| 39) |
Wise, K.C., Manna, S.K., Yamauchi, K., Ramesh, V., Wilson, B.L., Thomas, R.L., Sarkar, S., Kulkarni, A.D., Pellis, N.R. and Ramesh, G.T. : Activation of nuclear transcription factor-kappaB in mouse brain induced by a simulated microgravity environment. In Vitro Cell Dev. Biol. Anim., 41, 118-123, 2005. |
| 40) |
Xu, D., Perez, R.E., Rezaiekhaligh, M.H., Bourdi, M. and Truog, W.E. : Knockdown of ERp57 increases BiP/GRP78 induction and protects against hyperoxia and tunicamycin-induced apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol., 297, L44-51, 2009. |
| 41) |
Yasuhara, T., Hara, K., Maki, M., Matsukawa, N., Fujino, H., Date, I. and Borlongan, C.V. : Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience, 149, 182-191, 2007. |
| 42) |
Yoshimura, T., Kawano, Y., Arimura, N., Kawabata, S., Kikuchi, A. and Kaibuchi, K. : GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell, 120, 137-149, 2005. |