原 著
Buoyancy Induced Decrease of Maximal M-wave Amplitude in Human Soleus Muscle during Standing
Terumasa Takahara1, Hidetaka Yamaguchi2, Kazutoshi Seki3 and Sho Onodera1
1Faculty of Health Science and Technology, Kawasaki University of Medical Welfare, Kurashiki
2KIBI International University of Health Welfare & Human Performance, Takahashi
3Department of Hospital and Welfare Service, University of Marketing and Distribution Science, Kobe
ABSTRACT
The purpose of the present study was to compare the maximal M-wave (Mmax) amplitude in soleus muscle (SOL) during standing on land and that of in water, and investigate the effects of buoyancy. Nine healthy males were tested within a single day under two randomly administered conditions; (1) standing on land and (2) standing in water. H-reflex was elicited in SOL during standing in each condition by electrical stimulation to the tibial nerve in the popliteal fossa and surface electromyography (EMG) was recorded from SOL and tibialis anterior muscle (TA). Vertical ground reaction force (VGRF) and ankle joint angle were recorded before electrical stimulation. The average VGRF during standing in water was significantly lower (P<0.05) than that on land. However, ankle joint angle at electrical stimulations was not significantly different between both standing conditions. Furthermore, integrated EMG before electrical stimulation was not also significantly different between both standing conditions. Although latency, negative peak time and positive peak time of Mmax were not affected by standing conditions, Mmax amplitude during standing in water was significantly lower than that on land (P<0.05). These results suggest that inhibited Mmax amplitude in SOL during standing posture in water might be affected by the buoyancy that decrease the mechanical load to lower leg muscle.
(Received : 8 December, 2010 Accepted : 1 February, 2012)
Key words : maximal M-wave amplitude, buoyancy, mechanical load
INTRODUCTION
When the peripheral nerve is electrically stimulated at a high intensity, the direct motor response (from the point of stimulation to the neuromuscular junction; M-wave) can be detected by recording electromyography (EMG). The maximal M-wave (Mmax) amplitude indicates all α-motoneurons in the spinal cord of innervated muscles are excited. In the Hoffman (H) reflex studies, the H-reflex amplitude is expressed as a percentage of Mmax amplitude1,8,9,13,15,18). The submaximal M-wave amplitude is also used as the stationary of the electrical stimuli11,18). The M-wave pathway does not traverse the spinal cord, and hence it seems that M-wave amplitude is not influenced by the excitability changes within the central nerves systems (CNS).
Recent study, which showed changes in Mmax amplitude in soleus muscle (SOL) in response to alteration of the ankle joint position and/or of the strength of muscular contraction during isometric contraction, suggested that Mmax amplitude was influenced by afferent factors6). We also reported that Mmax amplitude in SOL was inhibited during standing compared to recumbent posture even with the same ankle joint angle19). In addition, our data support the relationship between Mmax amplitude and peripheral input, such as stretch reflex. There is the possibility that depression of Mmax amplitude affects the activities of spinal cord assessed by the maximal H-reflex amplitude (Hmax), normalized as the percentage of Mmax amplitude (Hmax/Mmax ratio). However, the mechanism responsible for the changes in Mmax amplitude is not clear yet.
In an aqueous environment, the characteristics of water cause the different physiological responses in human compared to land environment14). Body weight is decreased during standing in water by the buoyancy effect. The EMG activities in SOL during stationary standing in water were lower than those on land12). Therefore, buoyancy causes suppressive effect on the input from CNS. Furthermore, it is also considered that the posture-related mechanical load, applied to anti-gravity muscles, is relieved by buoyancy effect, which is associated with decreased body weight. The muscle activities of lower legs during human standing in water were reduced in proportion to the rise of immersion level from knee up to the neck9).
Since the peak-to-peak H-reflex amplitude is increased during head out water immersion compared to dry immersion5), it is also speculated that Mmax amplitude may be different between these conditions. Therefore, this study was performed to compare the Mmax amplitude in SOL during standing on land and in water, with difference in the mechanical load to muscle by the buoyancy. The hypothesis of this study was that mechanical load to lower leg is the factor of changes in Mmax amplitude in SOL.
METHODS
Subjects
Nine subjects (age : 22.0±2.0 years old, height : 174±4 cm, weight : 69.7±10.5 kg; mean±standard deviation, SD) participated in this study after providing written informed consent. All of them were healthy males without any neuromuscular disorders. The experiments were performed in accordance with the Declaration of Helsinki. The experiments were approved by the Ethics Committee on Human Experiments at Kawasaki University of Medical Welfare.
Experimental setup
Each subject was tested within a single day under two randomly administered conditions; (1) standing on land and (2) standing in water. Subjects stood with their eyes open and asked to stand at ease with their arms held comfortably to their sides and their feet separated at a distance corresponding to the shoulder width. The experiments in water were performed in a water tank (kas-4, Kz, Japan). The depth of water inside the tank was adjusted to the middle point between cleavage of knee joint and malleolus lateralis of each subject. The temperature of water was kept constant at 34.0°C.
Experimental procedures
Vertical ground reaction force (VGRF) in each standing condition was measured by using waterproof force platform (PZB 0009, Kistler, Japan), which was amplified using a charge meter (5015A-Kistler, Japan).
H-reflex was elicited in the right SOL muscle by electrical stimulation with single square pulse of 1-ms duration to the tibial nerve in the popliteal fossa (Stimulator : SEN-3301 and Isolator : SS202-J, Nihon Koden, Japan) during standing in each condition. The stimulation was delivered seven times randomly with more than 5-second interval without prediction to subjects.
Surface EMG was recorded in the right SOL and tibialis anterior muscle (TA) using Ag/AgCl bipolar electrodes (5 mm diameter, 20 mm interelectrode distance; Nihon Koden, Japan). EMG signals were amplified (JB-210J, Nihon Koden, Japan) and filtered (15-10,000 Hz). In order to decrease the interelectrode resistance, the skin was shaved, rubbed with sand paper and clean with alcohol before attaching the electrodes. The electrodes were covered with water resistant adhesive microfilms. The interelectrode resistances were less than 5 kΩ before and after experiment.
Ankle joint angle of right leg during electrical stimulation was recorded using electrical goniometer (SG 150/W, Biometrics, United Kingdom). All of the signals were digitized via the AD converter (Power Lab 800, AD instruments, Japan) with a 10-kHz sampling frequency, and stored on a personal computer (Power Book G4, Macintosh, USA).
Analyses of EMG data
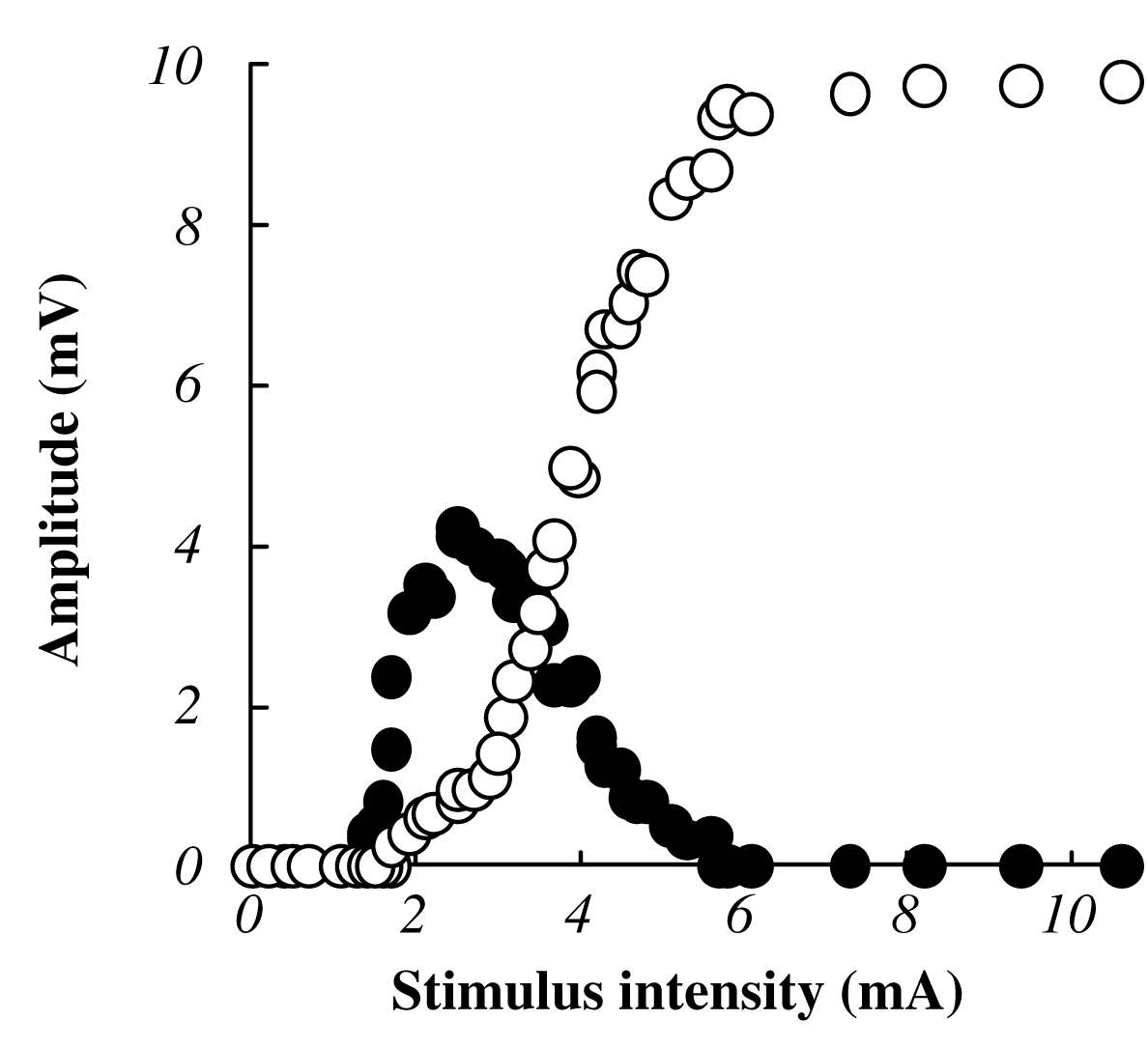
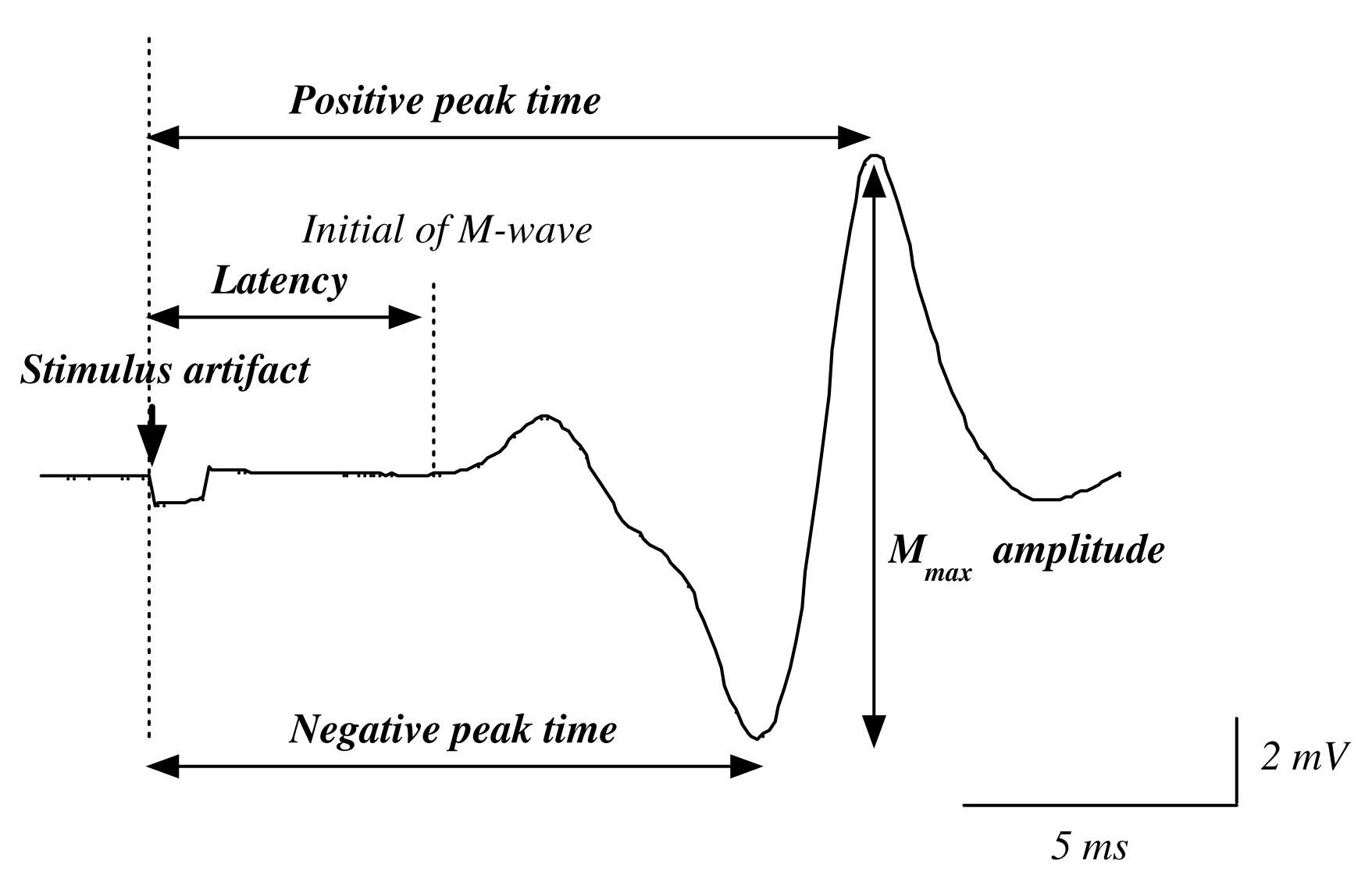
The Mmax amplitude was defined as the peak-to-peak amplitude of the M-wave at the time of verifying the peak out and disappearance of the H-reflex amplitude with the increases of stimulus intensity. Recruitment curves (Fig. 1) were constructed from data recorded at each standing condition to confirm the Mmax. After the full-wave rectification of EMG data, integral values of 50 ms prior to electrical stimulation were calculated as a background EMG activity. Three measurements of average Mmax included the time between the electrical stimulation and initiation of action potential (latency), the large negative peak (negative peak time), the large positive peak (positive peak time), and the peak-to-peak amplitude of the unrectified EMG signal (Fig. 2).
Statistical analysis
Data were expressed as means±SD. Differences between the means of each condition were analyzed using the paired t-test. The statistical significance was set to P<0.05. StatView (version 5.0, Japan) for Macintosh was used for the statistical analysis.
 |
|
 |
| Fig. 1 Typical example of H-reflex and M-wave recruitment curve in response to the electrical stimulation in one subject. Filled circles indicate the relationship between stimulus intensity and H-reflex amplitude. Open circles indicate the relationship between the stimulus intensity and M-wave amplitude. Amplitude was expressed as a percentage of the absolute value of Mmax at each standing condition. |
|
Fig. 2 The analysis of latency, negative peak time, positive peak time and amplitude of Mmax.
Latency: The time interval from stimulus artifact to initial of M-wave.
Negative peak time: Time interval from stimulus artifact to negative peak of M-wave.
Positive peak time: Time interval from stimulus artifact to positive peak of M-wave.
Amplitude: Peak-to-peak amplitude of M-wave. |
RESULTS
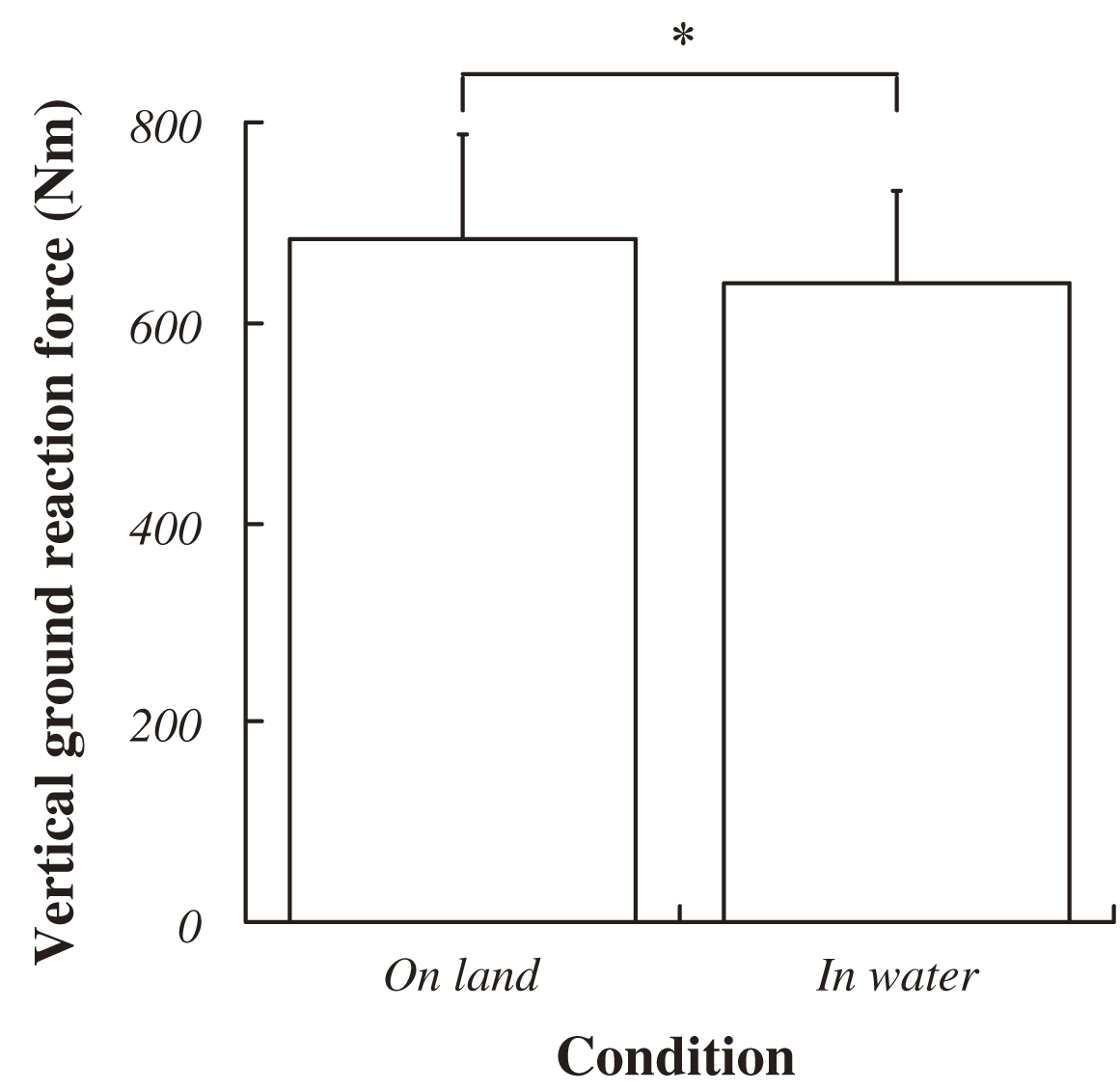
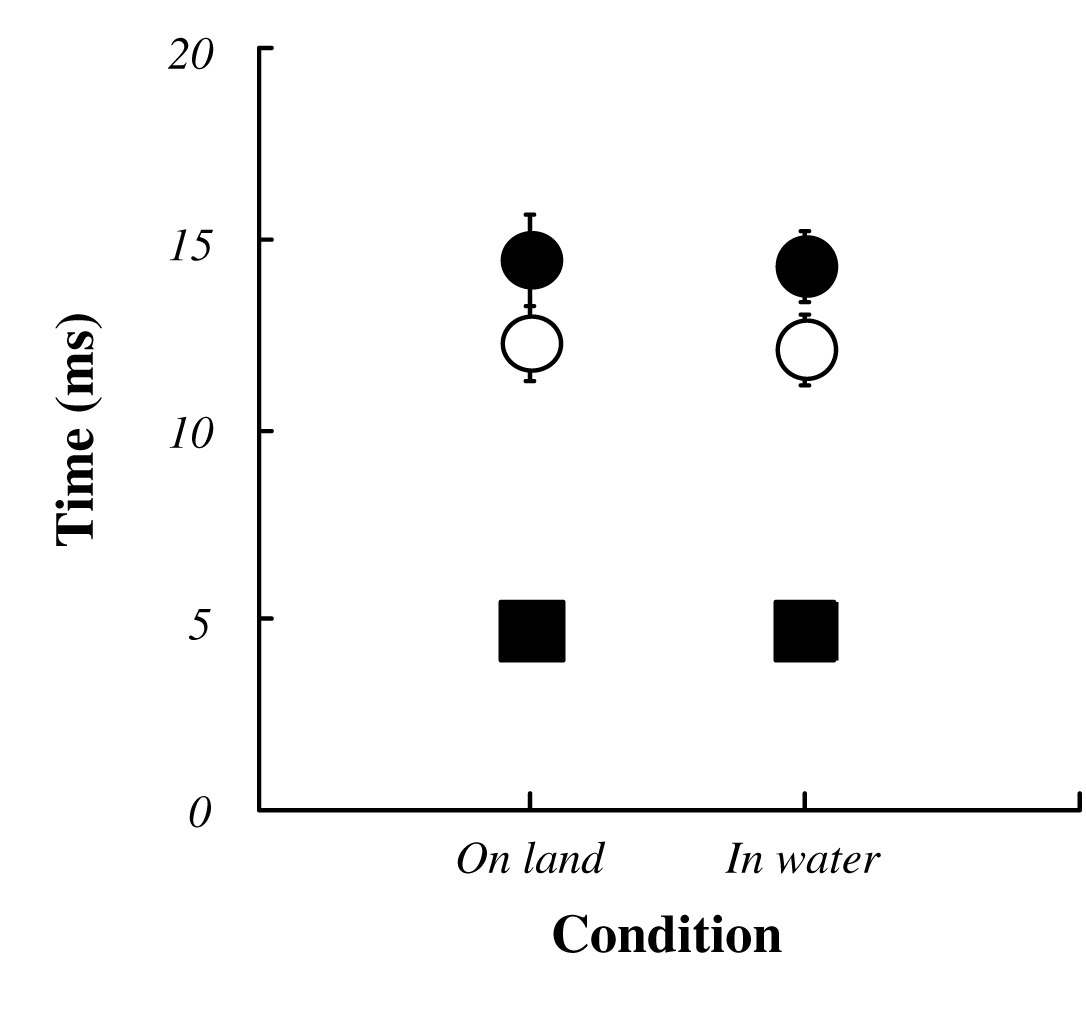
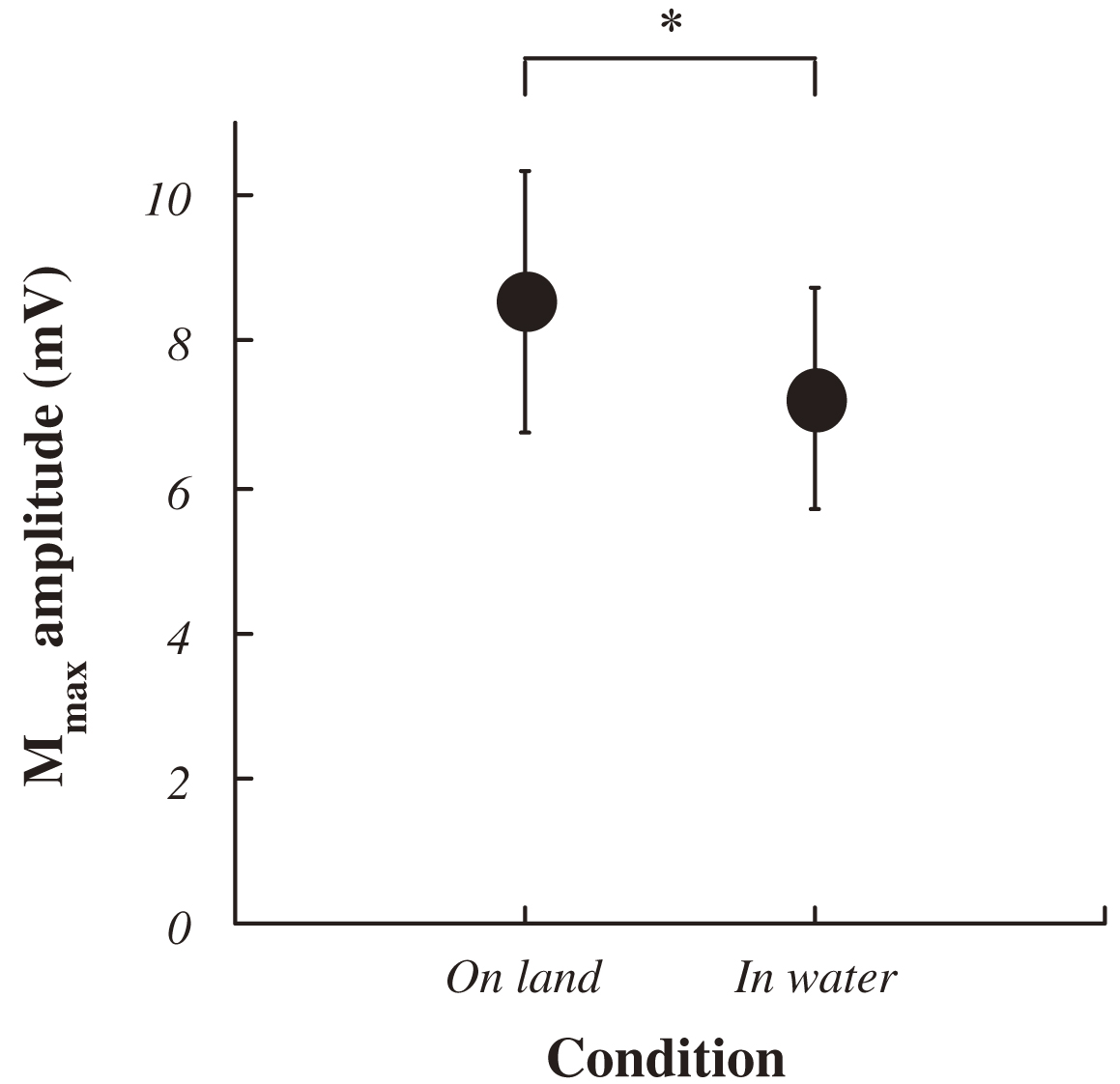
The average VGRF during standing in water was significantly lower than that on land (on land : 684±103 Nm, in water : 636±95 Nm; P<0.05, Fig. 3). Approximately 10% decreasing was observed during standing in water. Both ankle joint angles at electrical stimulation and integrated EMG in SOL and TA prior to electrical stimulation for 50 ms were no significant difference between standing on land and in water (Fig. 4, 5). Latency, negative peak time and positive peak time of Mmax were not affected by standing conditions (Fig. 6). Mmax amplitude during standing in water is significantly lower than that on land (on land : 8.55±1.82 mV, in water : 7.20±1.54 mV; P<0.05, Fig. 7).
 |
|
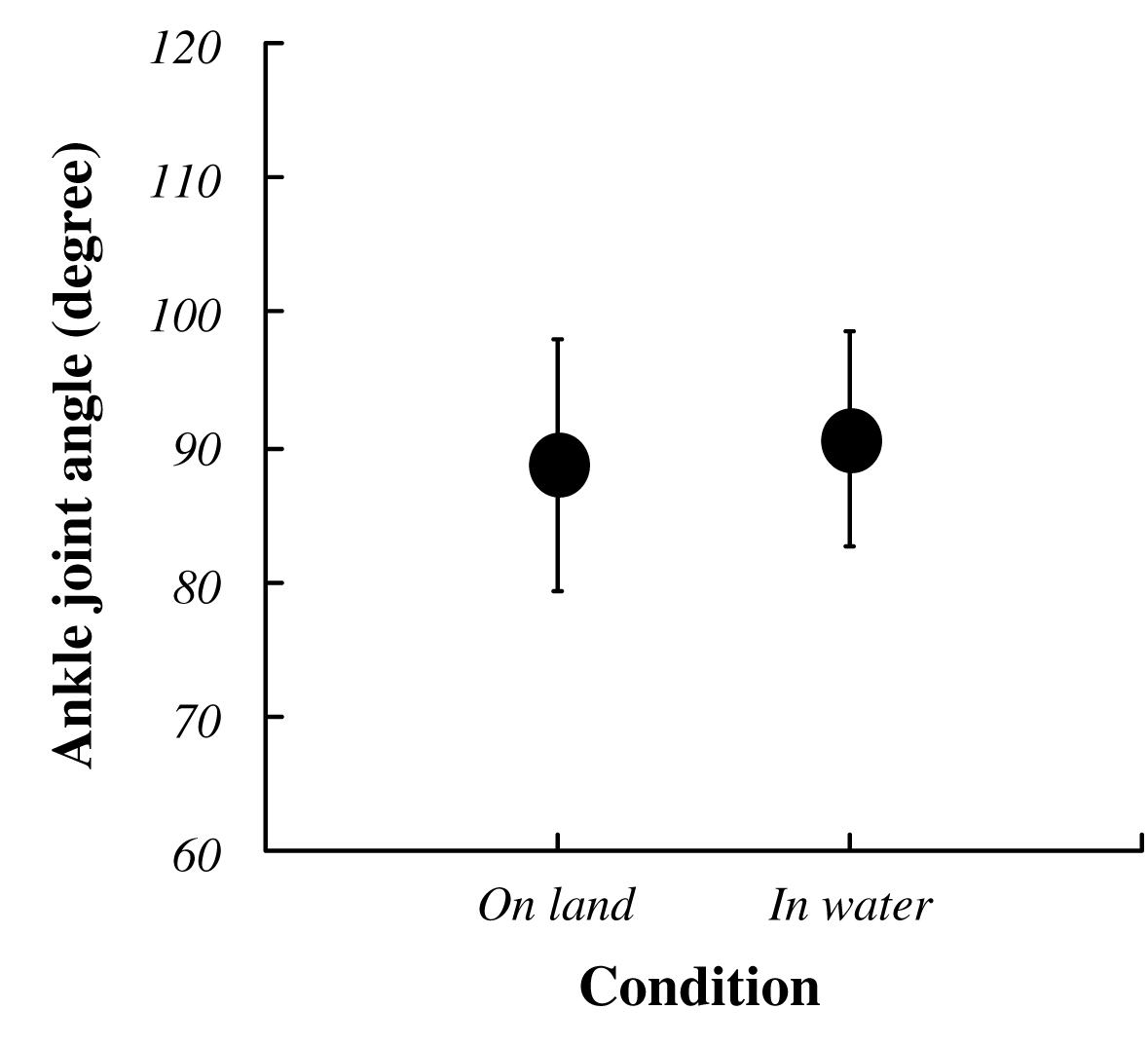 |
Fig. 3 Changes in the Vertical Ground Reaction Force (VGRF) in each condition.
Data ponits are mean±SD at each standing conditions for 9 subjects. Asterisk indicates a significant difference at P<0.05. |
|
Fig. 4 Changes in the ankle joint angle in each condition.
Data ponits are mean±SD at each standing conditions for 9 subjects defined neutral ankle position at prone as 0 degree. |
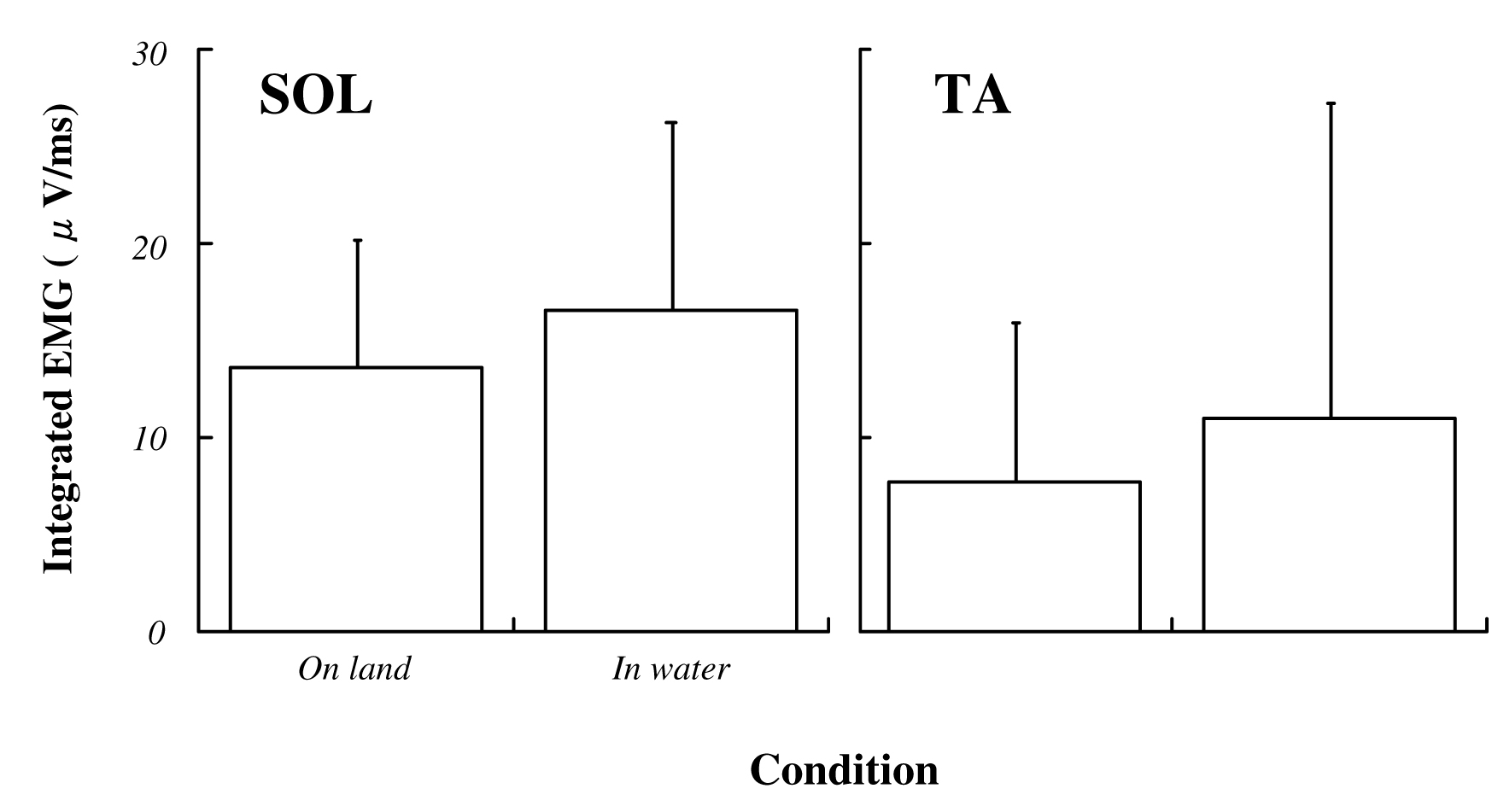 |
Fig. 5 Integrated EMG in soleus and tibialis anterior muscle for 50 ms before electrical stimulation in each condition.
Data ponits are mean±SD at each standing conditions. Data are expressed as a percentage of values at prone position.
SOL : soleus muscle and TA : tibialis anterior muscle |
 |
|
 |
| Fig. 6 The latency (filled squares), negative peak time (open circles) and positive peak time (filled circles) of the Mmax in each condition. |
|
Fig. 7 Changes in the Mmax amplitude in each condition. Data ponits are mean±SD at each standing condition for 9 subjects expressed as a percentage of the absolute values at prone position measured before an experiment. Data points (mean±SD) are percentages of the absolute values at prone position measured before the experiment in 9 subjects. Asterisk indicates significant difference at P<0.05. |
DISCUSSION
The buoyancy, the viscosity, the water temperature and the water pressure are physical characteristics of the water. In an aqueous environment, it is considered that body might be affected by these physical stimuli2,7,9,20). In the present study, experimental condition was static, and set the water temperature to 34°C considered as the physiological neutral temperature. From these factors, it can be exclude the influences of the water temperature and the viscosity in the present study. Additionally, it was reported that Mmax amplitude decreased with the time course of an experiment3). In the present study, the time between attaching EMG and end of an experiment was less than 1 hour, and experimental condition was set at random. For these reasons, there was less possibility that time course of experiment influenced in the present study.
Hmax/Mmax ratio often used as an excitability of spinal cord was increased during standing than sitting8). Furthermore, Mmax is largest when the strength of muscular contraction is high and Hmax/Mmax ratio is also grater with the strength of contraction6). Therefore, these studies suggest that changes in the excitability of spinal cord assessed by Hmax/Mmax ratio is influenced and changes by decreasing or increasing of Mmax amplitude.
No significant difference is shown in latency, negative peak time and positive peak time of Mmax in the present study. Conduction of depolarization of H-reflex during cooling and warming is affected to Na+ valtage-gated channel16) and similar effect on the Mmax latency would have consider. However, regarding Mmax, it is suggested short distance between the stimulation site and recording site would be masked quickening or slowing of the conduction velocity4). This study demonstrated that at least buoyancy is not affect to conduction of depolarization such as latency, negative peak time and positive peak time of Mmax during standing.
It was considered that approximately 10% decrease of the average VGRF in water was caused the buoyancy. Mmax amplitude during standing in water was significantly decreased compared with that on land. Postural control of human performed to adjust the activities of the anti-gravity muscles reflexively and voluntarily by affected from visual, vestibular and somatosensory systems17). Motoneuron pool of anti-gravity muscles is affected by somatosensory inputs from surface like the skin, and deep sensory like muscle spindle and tendon spindle. Decreasing of Mmax amplitude indicates the decreasing in the number of α-motor fibers that is recruited by for external stimuli. However, the number of all α-motoneurons of innervated muscles was not change. Microgravity environment enhances the H-reflex amplitude in SOL10), and it is suggested that the increased excitability of spinal cord might be associated with the removal of inhibitory mechanism related to graviception. The background EMG activities in SOL evaluated by the mean value of the full-wave rectified EMG over 100 ms to maintain standing posture in water is significantly decreased compared with that on land12). Thus, the decreasing of body weight causes the changes of efferent input from the CNS to lower legs. These data suggest that inhibition of the excitabilities in spinal cord might occur in case of decreasing body weight induced by the buoyancy. Slight decreasing of body weight might not influence the CNS during standing posture, because integrated EMG in both SOL and TA prior to electrical stimulation for 50 ms was no change between the conditions set the depth of water inside a tank at less than knee joint. There is no significant difference on ankle joint angle between standing on land and in water in this study, suggest afferent input from the muscle spindle generated by length change of SOL is not change. However, it is considered that mechanical load of SOL is reduced by the reduced body weight. Therefore, afferent input from deep sensory like the muscle spindle and tendon spindle might affect Mmax amplitude. Although it has been generally accepted that Mmax amplitude is no change in several conditions8,10,12,15), our results suggests that at least it needs to confirm Mmax amplitude when the experimental conditions (stimulus intensity, structures etc.) in H-reflex studies are decided.
The postural adjustment by the activity of the lower limbs muscle shown in upright posture is not performed shown in sitting position or recumbent position, and Mmax amplitude during standing position is decreased compared to prone position19). Mmax amplitude in SOL might be decreased when the lower limbs muscle contributes in the postural adjustment. Briefly, it is considered that Mmax amplitude would be increased at standing in water with the decreasing of gravitational force for lower legs compared to that on land. However, Mmax amplitude during standing in water is inhibited compared to that on land in the present study. These contradictory results suggest that the influences of Mmax amplitude have several factors, and mechanical load to lower limb is just an important factor.
CONCLUSION
Mmax amplitude in SOL is inhibited by standing posture in water which influences buoyancy and that might induced the decrease of mechanical load to lower leg muscle.
ACKNOWLEDGEMENT
We would like to express our gratitude to Hiromi Yano, Ph.D (Department of Health and Sports Science, Kawasaki University of Medical Welfare) for advice on this research and for his generous help in writing of the English manuscript.
REFERENCES
| 1) |
Armstrong, W.J., Nestle, H.N., Grinnell, D.C., Cole, L.D., Van Gilder, E.L., Warren, G.S. and Capizzi, E.A. : The acute effect of whole-body vibration on the hoffmann reflex. J. Strength. Cond. Res., 22, 471-476, 2008. |
| 2) |
Choukroun, M.L. and Varene, P. : Adjustments in oxygen transport during head-out immersion in water at different temperatures. J. Appl. Physiol., 68, 1475-1480, 1990. |
| 3) |
Crone, C., Johnsen, L.L., Hultborn, H. and Orsnes, G.B. : Amplitude of the maximum motor response (Mmax) in human muscles typically decreases during the course of an experiment. Exp. Brain Res., 124, 265-270, 1999. |
| 4) |
Dewhurst, S., Riches, P.E., Nimmo, M.A. and De Vito, G. : Temperature dependence of soleus H-reflex and M wave in young and older women. Eur. J. Appl. Physiol., 94(5-6), 491-499, 2005. |
| 5) |
Egawa, K., Oida, Y., Kitabatake, Y., Mano, T., Iwase, S., Kamiya, A. and Michikami, D. : Effect of Weight Bearing on the Soleus H-reflex During Upright Standing Under the Head-out Water Immersion Condition in Humans. Environ Med., 47, 81-84, 2003. |
| 6) |
Frigon, A., Carroll, T.J., Jones, K.E., Zehr, E.P. and Collins, D.F. : Ankle position and voluntary contraction alter maximal M waves in soleus and tibialis anterior. Muscle. Nerve., 35, 756-766, 2007. |
| 7) |
Gabrielsen, A., Johansen, L.B. and Norsk, P. : Central cardiovascular pressures during graded water immersion in humans. J. Appl. Physiol., 75, 581-585, 1993. |
| 8) |
Hayashi, R., Tako, K., Tokuda, T. and Yanagisawa, N. : Comparison of amplitude of human soleus H-reflex during sitting and standing. Neurosci. Res., 13, 227-233, 1992. |
| 9) |
Mano, T., Iwase, S., Yamazaki, Y., Saito, M. : Sympathetic nervous adjustments in man to simulated weightlessness induced by water immersion. J. Uoeh., 7, 215-227, 1985. |
| 10) |
Miyoshi, T., Nozaki, D., Sekiguchi, H., Kimura, T., Sato, T., Komeda, T., Nakazawa, K. and Yano, H. : Somatosensory graviception inhibits soleus H-reflex during erect posture in humans as revealed by parabolic flight experiment. Exp. Brain Res., 150, 109-113, 2003. |
| 11) |
Motl, R.W. and Dishman, R.K. : Acute leg-cycling exercise attenuates the H-reflex recorded in soleus but not flexor carpi radialis. Muscle. Nerve., 28, 609-614, 2003. |
| 12) |
Nakazawa, K., Miyoshi, T., Sekiguchi, H., Nozaki, D., Akai, M. and Yano, H. : Effects of loading and unloading of lower limb joints on the soleus H-reflex in standing humans. Clin. Neurophysiol., 115, 1296-1304, 2004. |
| 13) |
Ogawa, T., Kim, G.H., Sekiguchi, H., Akai, M., Suzuki, S. and Nakazawa, K. : Enhanced stretch reflex excitability of the soleus muscle in experienced swimmers. Eur. J. Appl. Physiol., 105, 199-205, 2009. |
| 14) |
Onodera, S., Miyachi, M., Nishimura, M., Yamamoto, K., Yamaguchi, H., Takahashi, K., In, J.Y., Amaoka, H., Yoshioka, A., Matsui, T. and Hara, H. : Effects of water depth on abdominal [correction of abdominails] aorta and inferior vena cava during standing in water. J. Gravit. Physiol., 8, 59-60, 2001. |
| 15) |
Patikas, D.A., Kotzamanidis, C., Robertson, C.T. and Koceja, D.M. : The effect of the ankle joint angle in the level of soleus Ia afferent presynaptic inhibition. Electromyogr. Clin. Neurophysiol., 44, 503-511, 2004. |
| 16) |
Rutkove, S.B. : Effects of temperature on neuromuscular electrophysiology. Muscle Nerve., 24(7), 867-882, 2001. |
| 17) |
Schieppati, M. : The Hoffmann reflex : a means of assessing spinal reflex excitability and its descending control in man. Prog. Neurobiol., 28, 345-376, 1987. |
| 18) |
Seki, K., Yamaguchi, H. and Onodera, S. : Responses of the latent time of H wave in human gastrocnemius muscle to arm crank exercise. J. J. Aerospace Env. Med., 45, 99-104, 2009. |
| 19) |
Takahara, T., Yamaguchi, H., Seki, K. and Onodera, S. : Posture induced changes in the Mmaximal M-wave amplitude and the H-reflex amplitude. Kawasaki. J. Med. Wel., 16(2), 50-56, 2011. |
| 20) |
Weston, C.F., O’Hare, J.P., Evans, J.M. and Corrall, R.J. : Haemodynamic changes in man during immersion in water at different temperatures. Clin. Sci. (Lond.), 73, 613-616, 1987. |
Send correspondence to:
Faculty of Health Science and Technology, Kawasaki University of Medical Welfare, 288 Matsushima, Kurashiki City, Okayama 701-0193, Japan
Terumasa Takahara
TEL : +81-86-462-1111 (ex 54531)
FAX : +81-86-464-1109
E-mail : w8508003@kwmw.jp






