 |
| Fig.1. The adductor longus muscle of right hindlimb in Wistar Hannover rat. |
宇宙航空環境医学 Vol. 46, No. 2, 21-28, 2009
原 著
Neural and/or Mechanical Responses of Adductor Longus Muscle to Exposure to Microgravity in Wistar Hannover Rats
Takashi Ohira1, Masahiro Terada1, Fuminori Kawano2, Naoya Nakai2, Toshimasa Ochiai3, Jun-ichiro Gyotoku3, Akihiko Ogura1 and Yoshinobu Ohira1, 2
Graduate School of 1Frontier Biosciences and 2Medicine, Osaka University
3Space Systems Designing Section, Mitsubishi Heavy Industries, Ltd., Kobe Shipyard & Machinery Works
ABSTRACT
Mechanical and/or neural responses of adductor longus (AL) muscle to exposure to micro-(μ-G) and/or hyper-gravity were studied in 5-week old male Wistar Hannover rats. Changes of gravity levels were created by the parabolic flight performed by a jet airplane. The electromyogram (EMG) activity of AL and the response of hip joints were analyzed simultaneously. The lengths of outer three sides in AL were also measured to estimate the length of muscle fibers at the rostral and caudal region, by fixing the muscle at the specific angles equivalent to those during either exposure to μ-G or a sedentary quadrupedal prone position on the floor using 4% buffered-formaldehyde. The EMG was tonic on the floor at 1-G and the integrated EMG activity level during quadrupedal position was greater in the caudal than the rostral region. The EMG activity decreased and activity patterns were changed from tonic to phasic during μ-G exposure. The hip joints were abducted to 60-70° and extended toward backward to 130-140° during exposure to μ-G. The estimated fiber length in the caudal side was shortened 25% during exposure to μ-G. That in the rostral region was even stretched by 13%, on the contrary. It was indicated that responses of the properties of AL to gravity levels were closely related to the region-specific mechanical and/or neural activities, being the caudal region more responsive. Further, the responses of neural muscular activities to exposure to μ-G were not affected significantly by the prior gravity level during the ascending phase of parabolic flight.
(Received:10 August, 2009 Accepted:8 September, 2009)
Key words:Wistar Hannover rats, adductor longus muscle, gravity level, electromyogram activity, fiber length
INTRODUCTION
Atrophy and shift of phenotype toward fast twitch type, particularly in slow-twitch soleus muscle, in response to gravitational unloading are well-reported (2-4, 9, 11, 12, 15, 20, 21). One of the causes for such phenomena is a decrease in neural activity of muscle, estimated by electromyogram (EMG) recording (1, 5, 9, 15). Another factor is the reduction of mechanical stress caused by a passive shortening of muscle fibers due to plantarflexion of ankle joints (5, 6, 9, 12, 18). It was reported that the tension development at the distal tendon of the soleus muscle was minimal because of the plantarflexion of ankle joints during hindlimb suspension of rats (5, 6).
It was reported that atrophy and shift of fiber phenotype toward fast twitch type were also induced in adductor longus (AL) muscle following gravitational unloading, caused by hindlimb suspension, in Wistar Hannover rats (10). However, it is not known how the slow-twitch AL muscle of rats respond to altered level of gravity. Therefore, the current study was performed in order to test the hypothesis that the regulation of the properties in AL muscle is also closely related to the level of neural and/or mechanical stress. The changes of gravity levels were created by the parabolic flight of a small jet airplane. And the responses of EMG and morphological properties of AL muscle in conscious rats were studied.
Although the parabolic flight can create an actual μ-G environment, the effect of prior hypergravity is the serious concern on any physiological responses seen in μ-G. Therefore, effects of prior three different gravity levels on the EMGs in μ-G environment were also studied in the present study.
METHODS
The experiment was performed using male Wistar Hannover rats with mean body weight of 150±3 (Mean±SEM) g. All experimental procedures were conducted in accordance with the Japanese Physiological Societies Guide for the Care and Use of Laboratory Animals. This study was also approved by the Committee on Animal Care and Use at Osaka University and Japan Aerospace Exploration Agency. All surgeries were performed under aseptic conditions with the rats under deep anesthesia (sodium pentobarbital, 5 mg/100 g body weight, i.p.).
Analyses of the electromyogram activities
i) Electrode implantation
Electrode implantation was performed as was reported elsewhere (1, 7, 8, 15, 19). Briefly, a skin incision in rat, anesthetized with i.p. injection of sodium pentobarbital (5mg/100g body weight), was made along the sagittal suture of the skull after shaving and cleaning with betadine. The exposed skull was dried and a head plug connector was firmly anchored to the skull using both screws and dental cement. Five enamel-coated constantan wires with 80-μm diameter were led subcutaneously from the connector to the back region.
The right AL muscle was exposed keeping the blood and nerve supplies intact (Fig.1). Bipolar electrodes were implanted into the caudal and rostral region of AL. The wires were inserted by threading individually through a 27-gauge hypodermic needle being passed through the muscle individually. The needle was carefully withdrawn and the insulated wire was stripped (-1mm). The section of the wire, with the insulation removed, was implanted into the mid-belly region of the muscle. Two wires were inserted in parallel with the muscle fibers (-2 mm apart). A wire for the common ground was also implanted at the back region. Each wire was secured with a suture at its entry and exit from the muscle, so that the stripped portion of the wire in the muscle was fixed. The exposed portions of wires were connected to a telemeter and preamplifier. Then, the skin was sutured.
 |
| Fig.1. The adductor longus muscle of right hindlimb in Wistar Hannover rat. |
ii) Recording of electromyogram
After 2 days of complete recovery from the anesthesia and surgery, EMG recordings were performed. The telemeter, preamplifier, and a battery (Dia Medical, Tokyo, Japan) were fixed to the rat by using a jacket (Lomir Biomedical Inc., Canada). A conscious male Wistar Hannover rat, kept in a plexiglass box of 30×30×30 (height) cm, was exposed to μ-G for approximately 20 sec in each parabolic flight. The EMGs were recorded throughout the 1-hr experiment per day. Changes in the environmental gravity levels were created by the parabolic flight performed by a jet airplane (Mitsubishi MU-300, Diamond Air Service, Nagoya, Japan). A total of 6 experiments were performed using one rat for each experiment. Within each experimental period, -12 parabolic or level flights were repeated. Various levels of gravity between 0- and 2.5-G were created during the ascending and descending phases of flight (Fig.2). In 4 experiments, the time course change of the gravity level was 1→1.5→2→μ→1.5→1-G. And one flight, in which the highest G level reached to 2.5-G, was added at the end of each experiment. Further, μ-G was created after the level flight in the remaining 2 experiments.
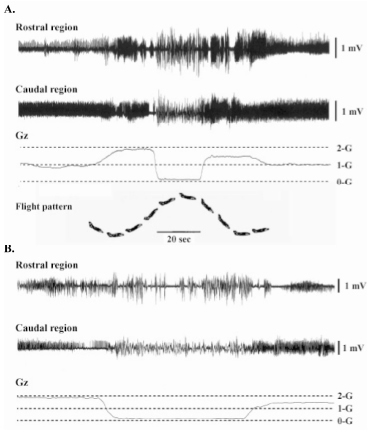 |
| Fig.2. The pattern of parabolic flight and changes in the gravity (Gz) and electromyogram activity of adductor longus muscle of a rat (A). Expanded patterns in A are shown in B. |
 |
 |
|
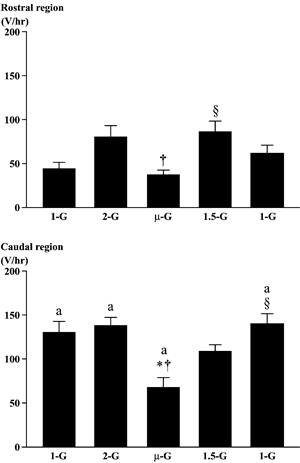
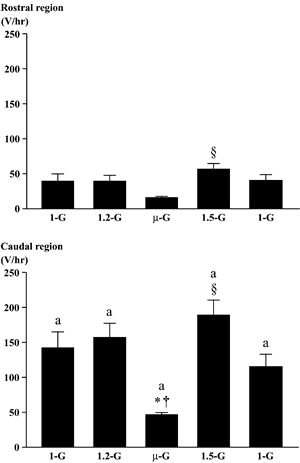
| Fig.3. Responses of the integrated electromyogram activities in the rostral and the caudal region of adductor longus muscle. Various gravity levels between 〜0(µ)-G and 〜2-G were created by a parabolic flight. Mean±SEM. The mean values were obtained from 40 parabolic flights. *, †, and §:p<0.05 vs. 1-G, 2-G, and µ-G, respectively. a:p<0.05 vs. the respective G level in the rostral region. | Fig.4. Responses of the integrated electromyogram activities in the rostral and the caudal region of adductor longus muscle. Various gravity levels between 〜0(µ)-G and 〜1.5-G were created by a level flight. Mean±SEM. The mean values were obtained from 24 level flights. *, †, and §:p<0.05 vs. 1-G, 1.2-G, and µ-G, respectively. a: p<0.05 vs. the respective G level in the rostral region. |
iii) Data analyses
The recordings of EMG were performed using a telemetry system (bandpass 10-2 kHz, DTT-201, Dia Medical System). The raw signals of EMG were sampled at 0.01 seconds of time constant and the bandwidth between 0 and 3kHz, and amplified (×1000) using AB-621G (Nihon Kohden, Tokyo) with 5 MΩ of input impedance and 60 dB of common mode rejection ratio. The amplified raw signals were processed by a PowerLab/16sp (ML795, AD Instruments, Australia), an analog-to-digital (A/D) converter, digitized at 2 kHz per channel, and stored on a computer. The integrated areas of EMGs were determined using a computer software package
(Chart v4.0.1, AD Instruments Inc., Australia).
Analyses of joint angles and shape of muscle
The responses of hip, knee, and ankle joints to changes of the gravity levels were video filmed throughout the 1-hr experiment. And the joint angles were analyzed later. These angles were compared with those during a sedentary quadrupedal prone position on the floor (n=5). The rat was sacrificed by over-dose injection of sodium pentobarbital (15mg/100g body weight) and AL muscles were exposed. The rat was placed on a styrene form board and the hip, knee, and ankle joints in each limb were fixed at the specific angles equivalent to those either during exposure to µ-G or a sedentary quadrupedal prone position on the floor, bilaterally. The AL muscles were covered with absorbent cottons containing 4% buffered-formaldehyde and were removed after 2 hrs of fixation. Subsequently, each muscle was sampled and the shape of muscle was analyzed by measuring the lengths of outer three sides.
Statistical analyses
All data were presented as means±SEM. Statistical significance was examined by two-way analysis of variance followed by Scheffé’s post hoc test. Differences were considered significant at the 0.05 level of confidence.
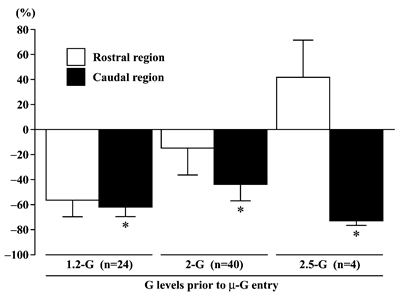 |
| Fig.5. The percent changes (relative to 1-G level) of integrated electromyogram activities in the rostral and caudal region of adductor longus muscle, when rat was exposed to µ-G environment from 1.2-, 2-, or 2.5-G. The total numbers of level or parabolic flights (n) are shown in the parenthesis. Mean±SEM. *: p<0.05 vs. 1-G (0%). |
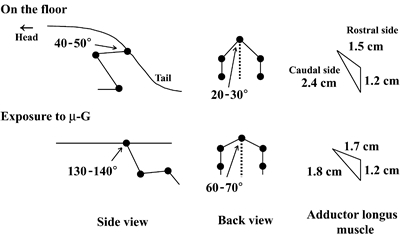 |
| Fig.6. The mean angles of hip joint of rat and perimeters of adductor longus muscle during quadrupedal resting position on the floor and exposure to µ-G. |
RESULTS
Electromyogram activity:
The EMG activity of AL was tonic during quadrupedal resting position on the floor at 1-G (Fig. 2) and the EMG activity level was greater in the caudal than the rostral region (p<0.05, Figs. 2-4). The EMG activity in the rostral, but not the caudal, region tended to increase when the rat was exposed to 2-G (p>0.05, Figs. 2 and 3). But, the increase of EMG level was not significant, when the gravity was increased to 2.5-G (data not shown). The integrated level of EMG in the caudal region decreased significantly vs. that at 1- and 2-G, when the rat was exposed to μ-G (p<0.05, Fig. 3). But that in the rostral region at μ-G was similar to the 1-G level, although the mean level was significantly less than the 2-G level (p<0.05). The EMG level in the rostral region increased, when the gravity level was increased to 1.5-G during the descending phase (p<0.05).
The increase of EMG level at 1.5-G in the caudal region was insignificant (p>0.05) by unknown reason, but returned to normal at 1-G (p<0.05).
The integrated levels of EMG in both regions were stable, when the gravity was slightly increased to 1.2-G before the entry to μ-G during the level flight (Fig. 4). But the EMG activity in both regions decreased, when the rat was exposed to μ-G. The reduction in the caudal region was statistically significant (p<0.05). Significant increase in EMG level was observed in both regions at 1.5-G during the descending phase (p<0.05). The patterns of EMG in both regions became phasic, when the rat was exposed to μ-G environment (Fig. 2).
Significant decrease in EMG level was induced only in the caudal region, when the rats were exposed to μ-G (Figs. 3-5). Even though the magnitude of the unloading-related decrease of EMG tended to be greater when the prior gravity level was 2.5-G, no statistical significance was noted between three different G levels (Fig. 5). The EMG level in the rostral region tended to be even increased (p>0.05) in μ-G environment.
Morphological responses of AL to µ-G exposure:
The mean hip joint angle from back view was approximately 20-30° and that from side view was approximately 40-50° during the sedentary quadrupedal position on the floor (Fig.6). The hip joints were abducted to 60-70° and extended toward backward to 130-140° during the exposure to µ-G. Pronounced change was observed in the shape of AL muscle, when the rat was exposed to µ-G environment. Due to the changes in posture and joint angles, the length of the outer side of caudal region decrease by -25%. But that of the rostral region was even increased (-13%).
DISCUSSION
Responses of AL muscle fibers in Wistar Hannover rats to changes of the gravity levels were studied. The results obtained from the current study clearly indicated that the responses of AL muscle to altered antigravity activities were region-specific. The EMG activities were generally greater in the caudal than the rostral region. The EMG activities in both regions decreased, when the rat was exposed to µ-G. But the response was pronounced in the caudal region. It was suggested these phenomena were closely related to the changes of muscle fiber length caused by the altered hip joint angle. To our knowledge, this is the first report showing that the responses of neural muscular activities to exposure to µ-G may not be affected significantly by the prior gravity level during the ascending phase of parabolic flight.
Atrophy of muscle fibers associated with the shift of phenotype toward fast twitch type is induced particularly in slow-twitch muscle, when gravitational loading is inhibited (2-4, 9, 11, 12, 15-17, 20, 21). Wang et al. (22) reported that the unloading-related atrophy and phenotype transformation of soleus muscle fibers in Wistar Hannover rats were prominent than those in other species (13). We also observed pronounced atrophy and shift of fiber phenotype in AL muscle of Wistar Hannover rats following hindlimb suspension (10).
The rostral region of AL was composed of -61% of fibers expressing type I myosin heavy chain (MHC) and 14 and 25% of fibers expressed type Ⅰ+ Ⅱ and Ⅱ MHC, respectively (10). On the contrary, -97% of fibers in the caudal region expressed pure type I MHC. The level of tonic EMG during quadrupedal resting position on the floor at 1-G was approximately 3 time greater in the caudal than the rostral region (Figs. 3 and 4). The difference in the percent distribution of fibers expressing type I MHC between the caudal and rostral region may be closely related to the pattern of neural activity at rest on the floor. Tonic EMG is typical in slow-twitch muscle. It was also reported that adult rats spent -78% of time at rest in a recumbent posture on the floor (14).
Further, the magnitudes of the responses of fiber properties to unloading were greater in the caudal than the rostral region (10). The data obtained in the present study suggest that such phenomena seen in the fibers from two different regions may be closely related to the specific neural responses to exposure to µ-G environment, although the duration of unloading was only -20 seconds in the present study. The EMG activity of AL decreased instantaneously in response to gravitational unloading by exposure to actual µ-G (Figs. 3 and 4), as was observed in soleus muscle (1, 5, 6, 15). The unloading-related decrease of EMG level was greater in the caudal than the rostral region (Figs. 3 and 4). Significant change in the pattern of muscular activity was also observed, when the rat was exposed to µ-G. The tonic EMG disappeared and phasic activity, which is typical in fast-twitch muscle, was noted in both regions (Fig. 2).
Clear gravity-associated responses were not seen in the level of EMG, when the gravity was increased during the ascending phase of parabolic flight. Although the integrated EMG level in the rostral region tended to increase at 2-G (p>0.05), that in the caudal region remained stable (Fig. 3). Significant change was noted in the posture during exposure to µ-G (Fig. 6). But the prone position was maintained when the gravity level was increased from 1-G to 2- or 2.5-G. The angles of hip, knee, and ankle joints were unchanged. It is suggested that the EMG activity did not change significantly, since the shape of AL muscle was also stable.
Mechanical load, applied to muscle, has been estimated by measurement of tension development of muscle by using a force transducer, placed at the distal tendon of soleus muscle and by analyzing the sarcomere length in vivo (5, 22). The passive and active force production by muscle was completely inhibited due to the plantarflexion-related shortening of muscle fibers and/or sarcomeres, when the rat was hindlimb suspended. It was also reported that the decrease of soleus EMG in response to hindlimb unloading was closely related to the passive shortening of muscle fibers or sarcomeres due to the plantarflexion of ankle joints, which decreases the mechanical load (5, 22).
Unloading-related change of the posture induced passive shortening of the caudal side and stretching of the rostral side of AL muscle in the present study. Although the sarcomere length was not measured, such responses clearly suggest that mechanical load in the caudal region may be decreased during exposure to µ-G. It is also speculated that load applied to fibers in the rostral region may be even increased slightly. These region-specific responses of mechanical load to the change in gravity level may be closely related to the alteration of EMG level.
CONCLUSION
Neural and mechanical responses of the caudal and the rostral region of AL muscle were studied, when the rats were exposed to hyper- and µ-G during the parabolic flight of a jet airplane. The EMG activities were tonic during prone position on the floor and were generally greater in the caudal than the rostral region regardless of the gravity level. The EMG activities in both regions decreased, when the rat was exposed to µ-G. But the response was pronounced in the caudal region. Further, the pattern of EMG changed to phasic during exposure to µ-G. It was suggested that these phenomena were closely related to the changes of muscle fiber length caused by the altered hip joint angle. The results obtained from the current study clearly indicated that the responses of neural activity, estimated by EMG level, and mechanical activity, estimated by the shape in AL muscle to altered gravity levels were region-specific, being the caudal region more responsive. Further, the responses of EMG activities to exposure to µ-G were not affected significantly by the prior gravity level.
ACKNOWLEDGEMENTS
This study was supported by the Grant-in-Aid for Scientific Research (S, 19100009) from Japan Society for the Promotion of Science.
REFERENCES
| 1) | Alford, E.K., Roy, R.R., Hodgson, J.A. and Edgerton, V.R.:Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hindlimb suspension. Exp. Neurol., 96, 635-649, 1987. |
| 2) | Allen, L., Yasui, W., Tanaka, T., Ohira, Y., Nagaoka, S., Sekiguchi, C., Hinds, W.E., Roy, R.R. and Edgerton, V.R.:Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J. Appl. Physiol., 81, 145-151, 1996. |
| 3) | Caiozzo, V.J., Baker, M.J., Herrick, R.E., Tao, M., and Baldwin, K.M.:Effects of spaceflight on skeletal muscle:mechanical properties and myosin isoform content of slow muscle. J. Appl. Physiol., 76, 1764-1773, 1994. |
| 4) | Edgerton, V.R. and Roy R.R.:Neuromuscular adaptations to actual and simulated spaceflight. In:Handbook of Physiology, Sect 4, Environmental Physiology, Ⅲ. The Gravitational Environment, Eds. by M.J. Fregly and C.M. Blatteis. Oxford University Press, New York, pp.721-763, 1996. |
| 5) | Kawano, F., Ishihara, A., Stevens, J.L., Wang, X.D., Ohshima, S., Horisaka, M., Maeda, Y., Nonaka, I., and Ohira, Y.:Tension- and afferent-input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am. J. Physiol.;Regul. Integr. Comp. Physiol., 287, R76-R86, 2004. |
| 6) | Kawano, F., Nomura, T., Ishihara, A., Nonaka, I. and Ohira, Y.:Afferent input-associated reduction of muscle activity in microgravity environment. Neuroscience, 114, 1133-1138, 2002. |
| 7) | Kawano, F., Nomura, T., Ishihara, A., Nonaka, I., and Ohira, Y.:Effects of chronic hindlimb suspension on landing performance in response to head-down drop in rats. Jpn. J. Aerospace Environ. Med., 39, 21-29, 2002. |
| 8) | Kawano, F., Wang, X.D., Lan, Y.B., Yoneshima, H., Ishihara, A., Igarashi, M. and Ohira, Y.:Hindlimb suspension inhibits air-righting due to altered recruitment of neck and back muscles in rats. Jpn. J. Physiol., 54, 229-242, 2004. |
| 9) | Ohira, M., Hanada, H., Kawano, F., Ishihara, A., Nonaka, I. and Ohira, Y.:Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn. J. Physiol., 52, 235-245, 2002. |
| 10) | Ohira, T.:Region-specific responses of adductor longus muscle to gravitational load-dependent activity in Wistar Hannover rats. Master’s thesis, Graduate School of Biosciences, Osaka University, 2009. |
| 11) | Ohira, Y.:Neuromuscular adaptation to microgravity environment. Jpn. J. Physiol., 50, 303-314, 2000. |
| 12) | Ohira, Y. and Edgerton, V.R.:Neuromuscular adaptation to gravitational unloading or decreased contractile activity. Adv. Exer. Sports Physiol., 1, 1-12, 1994. |
| 13) | Ohira, Y., Jiang, B., Roy, R.R., Oganov, V., Ilyina-Kakueva, E., Martin, J.F. and Edgerton, V.R.:Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J. Appl. Physiol., 73, 51S-57S, 1992. |
| 14) | Ohira, Y., Kawano, F., Roy, R.R. and Edgerton, V.R.:Metabolic modulation of muscle fiber properties unrelated to mechanical stimuli. Jpn. J. Physiol., 53, 389-400, 2003. |
| 15) | Ohira, Y., Nomura, T., Kawano, F., Sato, Y., Ishihara, A. and Nonaka, I.:Effects of nine weeks of unloading on neuromuscular activities in adult rats. J. Gravit. Physiol., 9, 49-60, 2002. |
| 16) | Ohira Y, Yoshinaga T, Nonaka I, Ohara M, Yoshioka T, Yamashita-Goto K, Izumi R, Yasukawa K, Sekiguchi C, Shenkman BS, and Kozlovskaya IB.:Histochemical responses of human soleus muscle fibers to long-term bedrest with or without countermeasures. Jpn. J. Physiol., 50, 41-47, 2000. |
| 17) | Ohira, Y., Yoshinaga, T., Ohara, M., Nonaka, I., Yoshioka, T., Yamashita-Goto, K., Shenkman, B.S., Kozlovskaya, I.B., Roy, R.R. and Edgerton, V.R.:Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol., 87, 1776-1785, 1999. |
| 18) | Riley, D.A., Ilyina-Kakueva, E.I., Ellis, S., Bain, J.L.W., Slocum, GR. and Sedlak, F.R.:Skeletal muscle fiber, nerve, and, blood vessel breakdown in space-flown rats. FASEB J., 4, 84-91, 1990. |
| 19) | Roy, R.R., Hutchison, D.L., Pierotti, D.J., Hodgson, J.A. and Edgerton, V.R.:EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol., 70, 2522-2529, 1991. |
| 20) | Thomason, D.B., Herrick, R.E. and Baldwin, K.M.: Activity influences on soleus muscle myosin during rodent hindlimb suspension. J. Appl. Physiol., 63, 138-144, 1987. |
| 21) | Thomason, D.B., Herric, R.E k., Surdyka, D. and Baldwin, K.M.:Time course of soleus myosin expression during hindlimb suspension and recovery. J. Appl. Physiol., 63, 130-137, 1987. |
| 22) | Wang, X.D., Kawano, F., Matsuoka, Y., Fukunaga, K., Terada, M., Sudoh, M., Ishihara, A. and Ohira, Y.:Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol., 290, C981-C989, 2006. |
Send correspondence to:
Section of Applied Physiology,
Graduate School of Medicine, Osaka University, 1-17 Machikaneyama-cho,
Toyonaka City, Osaka 560-0043, Japan
Yoshinobu Ohira
TEL and FAX:+81-6-6850-6032
E-mail: ohira@space.hss.osaka-u.ac.jp