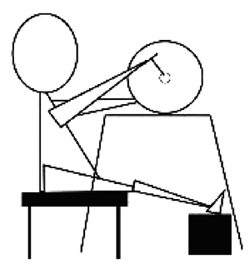
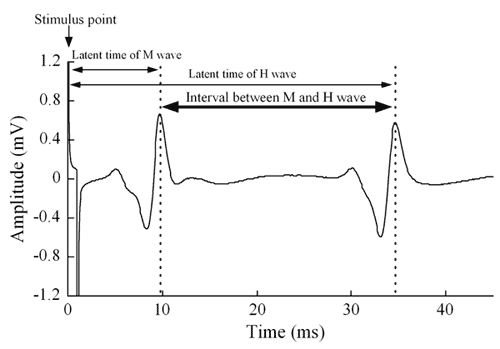
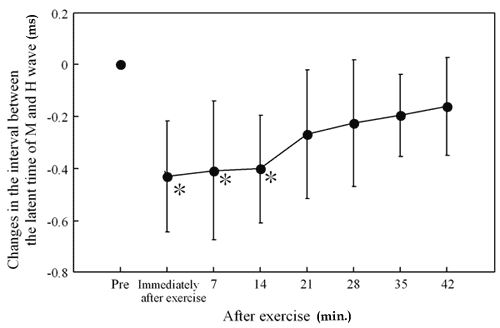
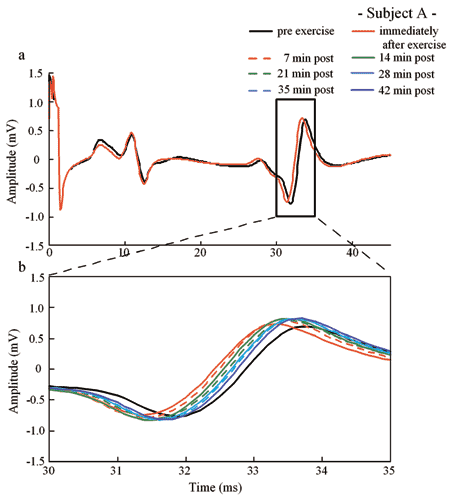
Responses of the Latent Time of H Wave in Human Gastrocnemius Muscle to Arm Crank Exercise
| 1) |
Arai, Y., Saul, J.P., Albrecht, P., Hartley, L.H., Lilly, L.S., Cohen, R.J. and Colucci, W.S.: Modulation of cardiac autonomic activity during and immediately after exercise. Am. J. Physiol., 256, 132-141, 1989. |
| 2) |
Bigland-Ritchie, B., Cafarelli, E. and Vollestad, N.K.: Fatigue of submaximal static contractions. Acta Physiol. Scand., 128, 137-148, 1986. |
| 3) |
deVries, H.A., Wiswell, R.A., Bulbulian, R. and Moritani, T.: “Tranquilizer effect of exercise, Acute effects of moderate aerobic exercise on spinal reflex activation level”. Am. J. Phys. Med., 60, 57-66, 1981. |
| 4) |
Dewhurst, S., Riches, P.E., Nimmo, M.A. and De Vito, G.: Temperature dependence of soleus H-reflex and M wave in young and older women. Eur. J. Appl. Physiol., 94, 491-499, 2005. |
| 5) |
Fallentin, N., Jorgensen, K. and Simonsen, EB.: Motor unit recruitment during prolonged isometric contractions. Eur. J. Appl. Physiol. Occup. Physiol., 67, 335-341, 1993. |
| 6) |
Feiereisen, P., Duchateau, J. and Hainaut, K.: MU recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp. Brain Res., 114, 117-123, 1997. |
| 7) |
Fuglevand, A.J., Zackowski, K.M., Huey, K.A. and Enoka, R.M.: Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J. Physiol., 460, 549-572, 1993. |
| 8) |
Hasking, G.J., Esler, M.D., Jennings, G.L., Dewar, E. and Lambert, G.: Norepinephrine spillover to plasma during steady-state supine bicycle exercise. Comparison of patients with congestive heart failure and normal subjects. Circulation, 78, 516-521, 1988. |
| 9) |
Hayes, K.C. and Sullivan, J.: Tonic neck reflex influence on tendon and Hoffmann reflexes in man. Electromyogr. Clin. Neurophysiol., 16, 251-61, 1976. |
| 10) |
Hiraoka, K. and Nagata, A.: Modulation of the soleus H reflex with different velocities of passive movement of the arm. Electromyogr. Clin. Neurophysiol., 39, 21-26, 1999. |
| 11) |
Hodgkin, A.L. and Katz, B.: The effect of temperature on the electrical activity of the giant axon of the squid. J. Physiol., 109, 240-249, 1949. |
| 12) |
Houtman, C.J., Stegeman, D.F., Van Dijk, J.P. and Zwarts, MJ.: Changes in muscle fiber conduction velocity indicate recruitment of distinct motor unit populations. J. Appl. Physiol., 95, 1045-1054, 2003. |
| 13) |
Kurata, H.: Long period constancy of threshold force of signal human motor units in voluntaryy contraction and its change induced by desire for micturtion. Jikeikai Med. J., 23, 103-111, 1974. |
| 14) |
Löscher, W.N, Cresswell, A.G. and Thorstensson, A.: Excitatory drive to the α-motoneuron pool during a fatiguing submaximal contraction in man. J. Physiol., 491(1), 271-280, 1996. |
| 15) |
Macefield, V.G. and Elam, M.: Comparison of the firing patterns of human postganglionic sympathetic neurones and spinal alpha motoneurones during brief bursts. Exp. Physiol., 89(1), 82-88, 2004. |
| 16) |
Mao, C.C., P. Ashby, M.W. and Mccrea, D.: Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp. Brain Res., 56, 341-350, 1984. |
| 17) |
Mazzocchio, A., Rossi, A. and Rothwell, J.C.: Depression of Renshaw recurrent inhibition by activation of corticospinal fibres in human upper and lower limb. J. Physiol., 481, 487-498, 1994. |
| 18) |
Misiazek, J.E., Brooke, J.D., Lafferty, K.B., Cheng, J. and Staines, W.R.: Long-lasting inhibition of the human soleus H-reflex pathway after passive. Brain Res., 677(1), 69-81, 1995. |
| 19) |
Moritani, T. and Muro, M.: Motor unit activity and surface electromyogram power spectrum during increasing force of contraction. Eur. J. Appl. Physiol. Occup. Physiol., 56, 260-265, 1987. |
| 20) |
Moritani, T., Muro, M. and Nagata, A.: Intramuscular and surface electromyogram changes during muscle fatigue. J. Appl. Physiol., 60(4), 1179-1185, 1986. |
| 21) |
Onodera, S., Morimoto, S. and Kasuga, N.: Relation Between the Functional of the Autonomic Nervous System and Voluntary Movement. Descente Sports Sci., 4, 265-270, 1983. |
| 22) |
Robinson, B.F., Epstein, S.E., Beiser, G.D. and Braunwald, E.: Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ. Res., 19, 400-411, 1966. |
| 23) |
Rowell, L.B. and O'Leary, D.S.: Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J. Appl. Physiol., 69, 407-418, 1990. |
| 24) |
RutKove, S.B.: Effects of temperature on neuromuscular electrophysiology. Muscle Nerve, 24, 867-882, 2001. |
| 25) |
Sakamoto, K. and Swie, Y.W.: EMG characteristics of low back and lower limb muscles during forward bending posture. Elecromyogr. Clin. Neurophysiol., 43, 335-347, 2003. |
| 26) |
Tanaka, S. and Nagata, A.: Motoneuron Pool on The Spinal Cord by The Measure of H wave Spectrum. A new development of time series analysis in medical and biological sciences., Hokkaido University Press, Hokkaido, pp. 703-716, 1996. |
| 27) |
Trimble, M.H. and Harp, S.S.: Postexercise potentiation of the H-reflex in humans. Med. Sci. Sports Exerc., 30(6), 933-941, 1998. |