FqσΒ«γw Vol. 44, No. 2, 59-64, 2007
Original
Responses of Vastus Lateralis Muscle Fibers to Cycling Tr aining with Different Loading Parameters in Human
M. Terada1, E. Lyubaeva3, T. Ohira1, A. Netreba3, F. Kawano2, H. Okabe4, T. Aoyama5, K. Imaizumi6, O. Vinogradova3, and Y. Ohira1C2
1Graduate School of Frontier Bioscience and 2Medicine, Osaka University
3State Scientific Center of Russian Federation-Institute for Biomedical
Problems
4Faculty of Letters, Kokushikan University
5Graduate School of Sports System, Kokushikan University
6Faculty of Human Sciences, Waseda University
ABSTRACT
@Responses of single muscle fibers of human vastus lateralis muscle to 8-week training with different loading parameters were studied in 22 male subjects, using a specially designed training machine with compulsory rotation of pedals induced by electrical engine. Three types of cycling were compared: 1) forward (concentric) with high velocity and 2) rearward (eccentric) compulsory rotation of pedals with high or 3) low velocity. Pedaling/rotation rate was 70 per min for high velocity groups and 35 rotations per min for low velocity group. Loads for high and low velocity groups were approximately 60% and 90% of the maximal alactic power accordingly. The repetitive exercise consisting of 1-min work periods divided by long (10 min) resting periods was used. Exercise bouts were repeated 5-7 times with 10-min resting periods. The training was performed twice a week for 8 weeks. Biopsy samples were taken from the vastus lateralis muscle before and after the training. Single muscle fibers were mechanically isolated and fiber cross-sectional area (CSA), myonuclear number, myonuclear CSA, and myonuclear domain were analyzed using a confocal microscope. Myonuclear CSA and myonuclear number per mm of fiber length did not change significantly after training in both regimes, although fiber CSA was increased significantly after eccentric training at either low or high intensity. Myonuclear domain size was also increased significantly by concentric and eccentric exercise. It was suggested that eccentric training was more effective for induction of muscle fiber hypertrophy. However, this phenomenon was not directly related to the increase of number and/or size of myonuclei.
(Received: 3 June, 2007 Accepted: 19 July, 2007)
Key words: concentric or eccentric bicycle exercise, vastus lateralis muscle, fiber size, myonuclei
INTRODUCTION
@It is well-known that the patterns of activity and/or mechanical load influence
the morphological, metabolic, and contractile properties of skeletal muscles.
For example, decreased use in response to gravitational unloading by exposure
to actual weightlessness12j or its simulation models, such as human bedrest1j
or hindlimb suspension of rats8j, causes a rapid atrophy in muscles that are
composed of slow-twitch fibers predominantly. On the contrary, an increased utilization
of muscle by removing the synergists, as well as the strength training, causes
a hypertrophy18j. Hypertrophy is also induced by stretching of muscle in
vivo4j
and in vitro17j. It is reported that the stretching prevents the unloading-related
atrophy of muscle fibers13C15C16j. However, it was also reported that exertional
muscle injury was induced in response to exhaustive eccentric contraction, in
which the forced active lengthening and/or stretching of muscles are involved,
even though such a phenomenon was not observed after concentric contraction19j.
@Unloading-related atrophy of muscle fibers is also associated with a decreased number of myonuclei14j. Further, the mean size of myonuclei is generally greater in atrophied muscle fibers than the normal controls, even though the DNA content in each nucleus is constant9C20j. It is suggested that protein synthesis by larger nucleus may be impaired. However, the precise mechanism responsible for the regulation of various properties of skeletal muscles is still unclear. Therefore, the responses of human skeletal muscle fibers to eccentric or concentric exercise training were investigated in the present study.
METHODS
@Twenty two healthy male volunteers participated in the current study. The mean
age, height, and weight were 21}1 (mean}SEM) years old, 177}1 cm, and 78}2 kg,
respectively. All subjects were evaluated clinically and were considered to be
in good physical condition. They were informed about the possible risks in attending
the exercise training and taking muscle biopsies. And a signed informed consent
was obtained from each subject. This study was approved by the Human Use Committees
at the Institute of Biomedical Problems (Moscow, Russia). All experimental procedures
were conducted in accordance with World Medical Association Declaration of Helsinki
(Ethical Principles for Medical Research Involving Human Subjects).
@The effects of bicycle training using a specially designed bicycle ergometer
with different pedaling parameters were investigated. Characteristic property
of the ergometer is compulsory rotation of pedals induced by electrical engine.
The difference of ergometer from the classic one is that the seat is not fixed
firmly, but slides along an inclined beam. As a result lower extremities carry
the load which is a part of the subject's weight and depends on the angle of
beam inclination. To proportion the pressure on the pedal, the subjects were
asked to maintain the position of the seat at the constant level.
@Three types of compulsory rotation were performed. Eight subjects performed
pedaling with forward compulsory rotation as the classical bicycling at the velocity
of 70 per min. Eight subjects performed pedaling with rearward compulsory rotation
at the same velocity. And the remaining 6 subjects performed rearward rotation
with the velocity of 35 per min. It was assumed that the concentric and eccentric
loads are applied to the knee and hip extensors during the forward and rearward
compulsory rotation, respectively. Thus, the groups were classified as 1) high
velocity-concentric, 2) high velocity-eccentric, and 3) low velocity-eccentric
training group, respectively. Training loads were chosen to fatigue muscles after
approximately 60 seconds of rotations. For high and low velocity groups, the
loads were approximately 60 and 90% of the maximal alactic power. The mean mechanical
power per one exercise day was the same in all three groups. The repetitive exercise
consisting of 5-7 working periods (1 min) divided by long resting periods (10
min) was used as daily training session. Two training sessions per week were
performed and the total duration of training was 8 weeks.
@Biopsy samples were taken from the vastus lateralis muscle of one leg before
and from the other leg after the training. The samples were immediately frozen
in liquid nitrogen and stored at |80 until analyses. Each sample was gradually
thawed to room temperature in a low-calcium relaxing solution, as describe previously2j.
Single muscle fibers were mechanically isolated in relaxing solution (at least
n=20 in each muscle). Each muscle sample was put into a tube filled with 500
μl relaxing solution and 500 μl of 100% glycerol. Relaxing solution contained
1.4 mM ethylene glycol bis (β-aminoethyl ether)-N, N, N,N-tetraacetic acid
(EGTA) and 8.6 mM MgCl2, 70 mM KCl, 80 mM imidazol, 4 mM adenosine-5-triphosphate
(ATP) disodium salt, and 11.3 mM phosphocreatine disodium salt in distilled water
(7.4 pH adjusted with propionic acid). These tubes were then stored in a freezer
(|20) over night. Next day, the muscle sample was placed on a glass plate,
coated with silicon, and fixed using pins, and was immersed in the relaxing solution.
Under microscope, a single fiber was separated using two pairs of tweezers and
was placed on a gelatin-coated glass slide and air-dried. Then, the slides were
stored in a freezer (|5) until analysed. Mechanical isolation of single fibers
has been shown to strip off the basal lamina of muscle fiber including satellite
cells2j.
@Immunohistochemistry and nuclear labeling
@The single muscle fiber segments were thawed and dried at room temperature.
The single fibers (n=20 for each muscle) was stained with 4.1~10|5 M
acridine orange, diluted in phosphate-buffered saline (PBS), for 5 min to label
the cytoplasm. And the myonuclei were stained with 1.5~10|7 M propidium iodide,
diluted in PBS, for 4 min. Immediately before the analysis, the fibers were
mounted in 50% glycerol with coverslips with gstrutsh of hardened nail polish
on the corners to minimize the fiber compression.
@Confocal microscopy
@A FV-300 confocal microscope with an argon laser (488 nm of peak wavelength,
Olympus) was used to analyze the fiber cross-sectional area (CSA), and the number
and CSA of the myonuclei. First, a maximum-intensity projection rotated orthogonally
to the long axis of the fiber was produced, and the fiber CSA was measured at
three non-overlapping regions, randomly chosen along the fiber length. The number
of myonuclei in the single fiber segment was counted. The length of the fiber
segment was also measured. Myonuclear domain3C6j was calculated by multiplying
the fiber CSA and length of the fiber segment and dividing by myonuclear number.
The fiber CSA and the length of fiber segment were normalized at a 2.5 μm sarcomere
length. The myonuclear number per mm of fiber length was calculated. The myonuclear
size was also measured in each myonucleus by Nomarski optic scan. To measure
the myonuclear size, the outline of the myonucleus was enclosed in the scanned
image and the area was measured. At least 30 myonuclei were checked for each
fiber.
@Statistical analyses
@All values were expressed as means}SEM. Significant differences were examined
by repeated measures of ANOVA followed by Scheffe's post hoc test. Differences
were considered significant at the 0.05 level of confidence.
RESULTS AND DISCUSSION
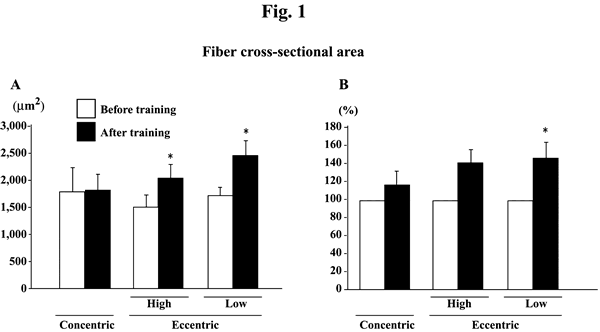
@The absolute fiber CSA was significantly increased after eccentric, but not
concentric, training at both high and low intensities (Fig. 1A, p<0.05). Since
the subject-specific fluctuations were noted in the levels obtained before the
initiation of exercise training, the percent change in each subject was also
calculated (Fig. 1B). The mean increase among 8 and 6 subjects, performed the
eccentric training with high or low velocity, was 40.0 (p>0.05) and 46.7%
(p<0.05), respectively. The mean percent increase of fiber CSA in 8 subjects
after concentric training was 16.6% (p>0.05).
 |
|
| Fig. 1. | Responses of the absolute (A) and the relative (to the pre-training
levels, 100%, B) fiber cross-sectional area of the vastus lateralis muscle
fibers to either concentric or eccentric training. Mean}SEM. n=8 in concentric
training group and n=8 and 6 in the eccentric training groups with high
and low velocity, respectively. *: p<0.05 vs. before training. |
@Myonuclear number per mm of fiber length did not change significantly in response to any training (Figs. 2A and B). Myonuclear CSA was not influenced by training, either (Figs. 3A and B). The myonuclear domain size was, generally, increased after the training (Figs. 4A and B). The absolute values of subjects, performed the eccentric training with high velocity, significantly increased (p<0.05). The mean relative increase after concentric or eccentric training with high or low velocity was 25.9% (p<0.05), 59.3% (p<0.05), and 29.6% (p>0.05), respectively.
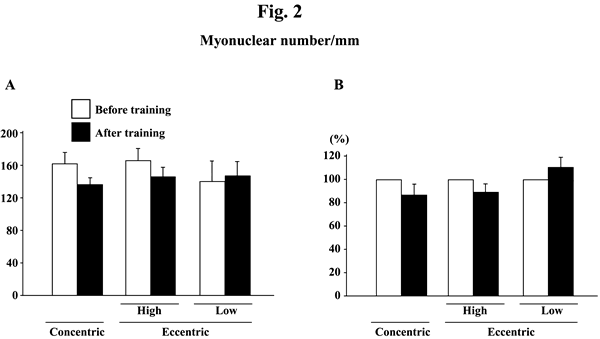 |
|
| Fig. 2. | Responses of the absolute (A) and the relative (to the pre-training levels, 100%, B) myonuclear number per mm of fiber length of the vastus lateralis muscle fibers to either concentric or eccentric training. Mean}SEM. n=8 in concentric training group and n=8 and 6 in the eccentric training groups with high and low velocity, respectively. |
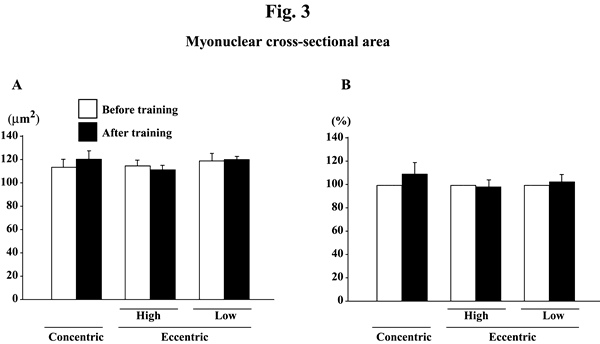 |
|
| Fig. 3.. | Responses of the absolute (A) and the relative (to the pre-training levels, 100%, B) myonuclear cross-sectional area of the vastus lateralis muscle fibers to either concentric or eccentric training. Mean}SEM. n=8 in concentric training group and n=8 and 6 in the eccentric training groups with high and low velocity, respectively. |
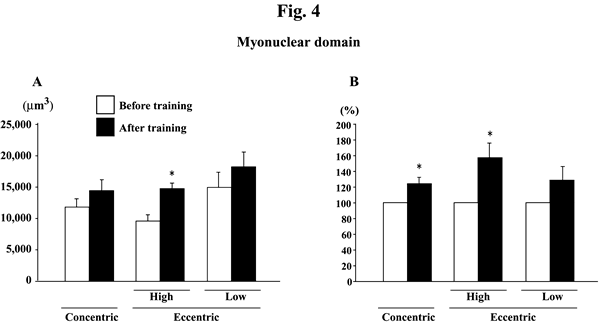 |
|
| Fig. 4. | Responses of the absolute (A) and the relative (to the pre-training levels, 100%, B) myonuclear domain of the vastus lateralis muscle fibers to either concentric or eccentric training. Mean}SEM. n=8 in concentric training group and n=8 and 6 in the eccentric training groups with high and low velocity, respectively. *: p<0.05 vs. before training. |
@LaStayo et al.10j and Higbie et al.7j reported that fiber CSA was increased
after eccentric training more than after concentric training. It was further
reported that eccentric contractions caused more profound changes in some aspects
of muscle function than concentric contractions11j. The fiber CSA was also
increased significantly by eccentric exercise but not by concentric exercise
in the present study.
@The degree of increase in myonuclear domain size tended to be greater after
high-velocity eccentric exercise than concentric exercise in the present study.
Chen et al.5j also reported that the expressions of cardiac ankyrin-repeated
protein, chemokine ligand 2, CCAAT/enhancer binding protein delta, IL-1 receptor,
tenascin C, and cysteine-rich angiogenic inducer 61 in vastus lateralis muscle
of human subjects, analyzed by quantitative real-time polymerase chain reaction,
were increased following the eccentric training. These results may suggest that
the function of myonuclei might be improved by eccentric exercise. Furthermore,
myonuclear CSA tended to increase slightly following the concentric, but not
eccentric, training (p>0.05). We previously reported that the smaller myonuclei
may have the greater function than the myonuclei with larger size9C20j. Thus,
it is also suggested that the protein synthesis by myonuclei might be more stimulated
by eccentric than concentric training.
@Significant atrophy of soleus muscle fibers was induced in response to 4-month
bedrest in male subjects, although the myonuclear number was stable15j. Therefore,
the myonuclear domain size was decreased after bedrest, suggesting that the function
of each myonucleus might be inhibited or that the breakdown of cytoplasmic proteins,
which may be unrelated to myonuclei, could be stimulated. The fiber atrophy was
prevented by passive stretching of soleus due to dorsiflexion of ankle joints.
These results suggest that the plasticity of morphological properties of muscle
fibers could be modulated by some mechanism (s), which is not necessarily directly
related to the function of myonuclei.
@In conclusion, the eccentric training was more effective for induction of muscle
fiber hypertrophy compared with the concentric training. However, this phenomenon
was not directly related to the changes of the number and/or size of myonuclei.
Beneficial effect of eccentric training is suggested, although it is also possible
to cause muscle injury if the work intensity is too severe19j.
ACKNOWLEDGEMENTS
@This investigation was sponsored by the Russian Ministry of Education and Sciences,
Governmental Contract No. 02.522.11.2004. This study was also carried out as
a part of Grant-in-Aid for Scientific Research (S, 19100009, Y.O.) from Japan
Society for the Promotion of Science, Grant-in Aid for Exploratory Research (18650188,
Y.O.) and for Young Scientists (B, 15700417, F.K.) from Ministry of Education,
Culture, Sports, Science and Technology, and Grant-in-Aid for the Academic Frontier
Project (Waseda University: 2005-2010) of the Ministry of Education, Culture,
Sports, Science and Technology (K.I.).
References
| 1) | Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294-305, 2004. |
| 2) | Allen DL, Monke SR, Talmadge RJ, Roy RR, and Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol 78: 1969-1976, 1995. |
| 3) | Allen DL, Roy RR, and Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22: 1350-1360, 1999. |
| 4) | Carson JA, Yan Z, Booth FW, Coleman ME, Schwartz RJ, Stump CS. Regulation of skeletal alpha-actin promoter in young chickens during hypertrophy caused by stretch overload. Am J Physiol 268: C918-C924, 1995. |
| 5) | Chen YW, Hubal MJ, Hoffman EP, Thompson PD, Clarkson PM. Molecular responses of human muscle to eccentric exercise. J Appl Physiol. 95: 2485-2494, 2003. |
| 6) | Hall ZW, and Ralston E. Nuclear domains in muscle cells. Cell 59: 771-772, 1989. |
| 7) | Higbie EJ, Cureton KJ, Warren GL III, Prior BM. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol. 81: 2173-2181, 1996. |
| 8) | Kawano F, Ishihara A, Stevens JL, Wang XD, Ohshima S, Horisaka M, Maeda Y, Nonaka I, Ohira Y. Tension- and afferent input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am J Physiol Regul Integr Comp Physiol 287: R76-R86, 2004. |
| 9) | Kawano F, Matsuoka Y, Oke Y, Higo Y, Terada M, Wang X, Nakai N, Fukuda H, Imajoh-Ohmi S, Ohira Y. Role(s) of nucleoli, phosphorylation of ribosomal protein S6 and/or HSP27 in the regulation of muscle mass. Am J Physiol Cell Physiol., In press. |
| 10) | LaStayo PC, Pierotti DJ, Pifer J, Hoppeler H, Lindstedt SL. Eccentric ergometry: increases in locomotor muscle size and strength at low training intensities. Am J Physiol Regul Integr Comp Physiol. 278: R1282-R1288, 2000. |
| 11) | Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond). 64: 55-62, 1983. |
| 12) | Ohira Y, Kawano F, Stevens JL, Wang XD, Ishihara A. Load-dependent regulation of neuromuscular system. J Gravit Physiol 11: 127-128, 2004. |
| 13) | Ohira Y, Yasui W, Roy RR, Edgerton VR. Effects of muscle length on the response to unloading. Acta Anat 159: 90-98, 1997. |
| 14) | Ohira Y, Yoshinaga T, Nomura T, Kawano F, Ishihara A, Nonaka I, Roy RR, Edgerton VR. Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number. Adv Space Res 30: 777-781, 2002. |
| 15) | Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita-Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, and Edgerton VR. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol 87: 1776-1785, 1999. |
| 16) | Ohira Y, Yoshinaga T, Yasui W, Ohara M, Tanaka T. Effects of hindlimb suspension with stretched or shortened muscle length on contractile properties of rat soleus. J Appl Biomech 16: 80-87, 2000. |
| 17) | Perrone CE, Fenwick-Smith D, Vandenburgh HH. Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J Biol Chem 270: 2099-2106, 1995. |
| 18) | Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol 102: 368-373, 2007. |
| 19) | Shellock FG, Fukunaga T, Mink JH, Edgerton VR. Exertional muscle injury: evaluation of concentric versus eccentric actions with serial MR imaging. Radiology 179: 659-664, 1991. |
| 20) | Wang XD, Kawano F, Matsuoka Y, Fukunaga K, Terada M, Sudoh M, Ishihara A, and Ohira Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am J Phyisol Cell Physiol 290: C981-C989, 2006. |
Send correspondence to:
@Y. Ohira, Ph.D.
@Section of Applied Physiology
@Graduate School of Medicine
@Osaka University
@Toyonaka City, Osaka 560-0043, Japan
@Phone & FAX: +81-6-6850-6032
@E-mail: ohira@space.hss.osaka-u.ac.jp