FqσΒ«γw Vol. 44, No. 1, 19-29, 2007
Original
Effects of Mechanical and/or Neural Stimuli on the Properties of Soleus Muscle Fibers in mdx Mice
Yong Bo Lan1*, Masahiro Terada2*, Yoko Higo3, Fuminori Kawano1,
Yoshikazu Matsuoka2, Xiao Dong Wang1, Naoya Nakai1, Yoshinobu Ohira1C2
Graduate School of 1Medicine and 2Frontier Biosciences, and 3School of Science, Osaka University
* Contributed equally.
ABSTRACT
@Roles of mechanical load and/or neural activity in the regulation of soleus
muscle fibers of mice were studied. Five-week-old male mdx and wild type mice
were separated into tenotomy (T), denervation (D), and T+D groups. The distal
tendons of the left plantarflexors (soleus, plantaris, and gastrocnemius) were
ablated in the T group. The left sciatic nerve was transected at the gluteal
region in the D group. For group T+D, both T and D were performed. The right
limb was kept intact as the normal control. Ambulation was allowed after the
surgery. Soleus muscle was sampled bilaterally 14 days after the surgery and
analyses were performed in cross-section of whole muscle. Fiber atrophy with
identical degree was induced in both wild type and mdx mice following each
treatment in the order of T+D>D>T. The magnitude of the atrophy was also similar
between the fibers with and without central nuclei. The percentage of fibers
expressing pure type I or II MHC in mdx mice was less than wild type mice,
and the distribution of fibers co-expressing type I and II MHC in mdx mice
was greater on the contrary. No significant changes in the distribution of
the fiber phenotype were observed following each treatment in wild type mice.
But the distribution of type I+II fibers in mdx mice was increased and that
of type II fibers decreased following D. Similar tendency was also observed
in wild type mice, but the changes were not significant statistically. The
degree of shift from type II to I+II was identical between fibers with and
without central nuclei. It was suggested that neural activity and mechanical
loading play some roles in the regulation of the mass and phenotype of soleus
muscle fibers in both wild type and mdx mice. It was further indicated that
the regulation of these properties were not related to the localization of
myonuclei.
(Received: 20 October, 2006 Accepted: 5 January, 2007)
Key words: Fiber size and/or phenotype, soleus of mdx and wild type mice, central nuclei, mechanical loading, neural activity
INTRODUCTION
@Duchenne muscular dystrophy is a fatal disease that is observed in 1 out of
3,500 males9j. It is caused by mutation of the dystrophin gene
that encodes an elongated 427 kDa protein associated with the plasma membrane
of skeletal and cardiac muscles3j. The characteristic of duchenne
muscular dystrophy is a progressive wasting and weakness of skeletal muscles
leading to death around the age of 20.
@The muscles of dystrophin-deficient (mdx) mice are characterized by cycles
of muscle fiber necrosis followed by regeneration10j. The absence
of dystrophin in muscle causes a dramatic secondary reduction of the dystrophin-associated
protein complex (DPC) at the plasma membrane (ΐ-dystroglycan and Ώ-sarcoglycan,
two major components of the DPC)33j. The lack of dystrophin in
mdx muscle causes a reduction in force-generating capacity of the muscle, and
increases its sensitivity to mechanical stress-induced injury following the
application of lengthening (eccentric) contraction. It is well-known that the
necrosis in skeletal muscle of the mdx mice is caused by the extreme influx
of calcium ion21C30C31C35j. Further, the regenerated fibers can
be easily identified, because they contain conspicuous internal (central) myonuclei4j.
@Inhibition of weight-bearing activity of hindlimbs of rat induces a pronounced
atrophy and shift towards a faster fiber phenotype in soleus muscle25j.
One of the causes for the unloading-related changes in muscle properties is
often attributed to a decrease in neuromuscular activation1C22C23j.
The electromyogram activity of soleus decreases in response to hindlimb unloading,
although these levels gradually increase and return to the normal levels within
1 to 2 weeks of suspension. In spite of the return to normal activation levels,
however, the soleus continue to atrophy23j, clearly indicating that
the direct cause of atrophy is not simply a decrease in the activation level
of a muscle.
@In addition to changes in activation associated with hindlimb unloading, development
of active and passive forces also decreases17C18C24j. During hindlimb
unloading, the ankle joints are chronically extended resulting in a passive
shortening of the ankle plantarflexors17C18C22C24C29j. Kawano et
al.17C18j reported minimal tension development at the distal tendon
of the soleus muscle in response to plantarflexion with the ankle joint maintained
at `140 to 160ί of extension. Thus, both the active and passive tension levels
of the plantarflexors would be expected to be relatively low, particularly
during the early phases (up to 2 weeks) after the initiation of hindlimb unloading17j.
@These data strongly indicate that the adaptations of muscles in normal animals
induced by hindlimb unloading are directly associated with the levels of mechanical
loading and/or activation. However, it is also suggested that the regulation
of various properties of skeletal muscles in response to loading and/or activation
cloud be different in mdx mice from other species. Therefore, the current study
was performed to investigate the responses of soleus muscle of mdx mice to
inhibition of either mechanical loading and/or neural activity. Denervation
and tenotomy were performed to minimize all neural activity-dependent and -independent
influences and any residual passive tension in the muscles, respectively.
MATERIALS AND METHODS
@All experimental procedures and animal care were conducted in accordance with
the Japanese and American Physiological Society Guide for the Care and Use
of Laboratory Animals. The study was also approved by the Animal Use Committee
at Graduate School of Medicine, Osaka University.
@Animal Care and Surgeries
@Five-week-old male C57BL/10-mdx Jic (dystrophin-deficient, mdx, n=15) and
C57BL/6J Jcl (wild type, n=15) mice were used in the present study. The mdx
and wild type mice were divided into the tenotomy (T), denervation (D), and
T+D (n=5 for each treatment in mdx and wild type mice, respectively) groups.
All mice were housed in a controlled environment with 12: 12 hr of light: dark
cycle and the temperature and humidity were maintained at `23ίC and `55%, respectively.
Two to three mice were housed in a cage with 11~20 cm and 11 cm height. Solid
food (CE-2, Nihon CLEA, Tokyo) and water were supplied ad libitum.
@Modified procedures reported by Jalubiec-Puka et al.13C14j and
Ohira et al.26j were used for the treatment with T, D, and/or T+D.
All surgeries were performed under anesthesia with i.p. injection of sodium
pentobarbital (0.5 mg/10 g body weight) on the first day of the experiment.
The distal tendons of the left soleus, plantaris, and gastrocnemius muscles
were ablated keeping the nerve supply and blood flow intact in the T group.
As for the D group, a skin incision was made at the left gluteal region and
the sciatic nerve was exposed and transected (1-2 mm). Both treatments were
performed for the T+D group. The skin was sutured and the animals were allowed
normal ambulation in the cage for 14 days. To eliminate the other effect except
neural and mechanical activity between individual mice, the hindlimb muscles
of the contralateral side were kept intact and served as the normal control.
@Muscle Preparation
@On the 14th day after surgery, the mice were sacrificed by overdose injection
of sodium pentobarbital (i.p., 0.5 mg/10 g body weight). The left and right
soleus muscles were sampled and cleaned of excess fat and connective tissue.
The muscles were pinned on a cork at an optimum length, frozen in isopentane
cooled with liquid nitrogen, and stored at |80ίC until the cross-sectional
analyses.
@Cross-Sectional Analyses
@Cross-sectional analyses was done by using the procedures described previously19C26j.
Briefly, the mid-portion of the frozen muscle was mounted on a cork by using
optimum cutting temperature (OCT) compound (Miles, IN, U.S.A) and 3 serial
cross-sections of the soleus muscle (10 Κm thickness) were cut in a cryostat
maintained at |20ίC. These sections were used for the analyses of myosin heavy
chain (MHC) expression, and the distribution of myonuclei at either central
and/or peripheral regions. The expression of MHC was analyzed by using monoclonal
antibodies specific for slow (type I) or fast (type II) MHC isoform, i.e.,
primary antibody, NCL-MHCs and NCL-MHCf (Novocastra Laboratories, UK), as described
previously19j. Briefly, the avidin-biotin immunohistochemical procedure
was used for the localization of primary antibody binding according to the
instructions for ABC kit (Vector Laboratories, Burlingame, CA, U.S.A.). Phosphate
buffered saline was used as a buffer for all immunoglobulin G-class primary
antibodies. The visualization for primary antibody binding site was performed
with diaminobenzidine tetrahydrochloride. The stained images were incorporated
into a computer. Number of primary antibody positive fibers was counted in
the whole muscle cross-section stained with antibodie specific for slow or
fast MHC. Fiber phenotypes were classified as type I, I+II, or II.
@The remaining section was stained with haematoxylin for 5 min at room temperature.
The fibers with or without central nuclei were identified by visual examination.
The images of the same area of each section were incorporated into a computer.
The stained images were digitized as grey-level pictures using image processing
software (Scion image). The fiber cross-sectional areas (CSAs) were analyzed
using Scion image. The fibers in each cross-section were matched with those
analyzed the myonuclear distribution in other section.
@Statistical Analyses
@All data were presented as mean}SEM. Statistical significance was examined
by two-way analysis of variance (ANOVA) followed by Scheffe's post hoc test.
Paired t-test was also performed for the comparison of the left and right muscle
in the same animal. Differences were considered significant at the 0.05 level
of confidence.
RESULTS
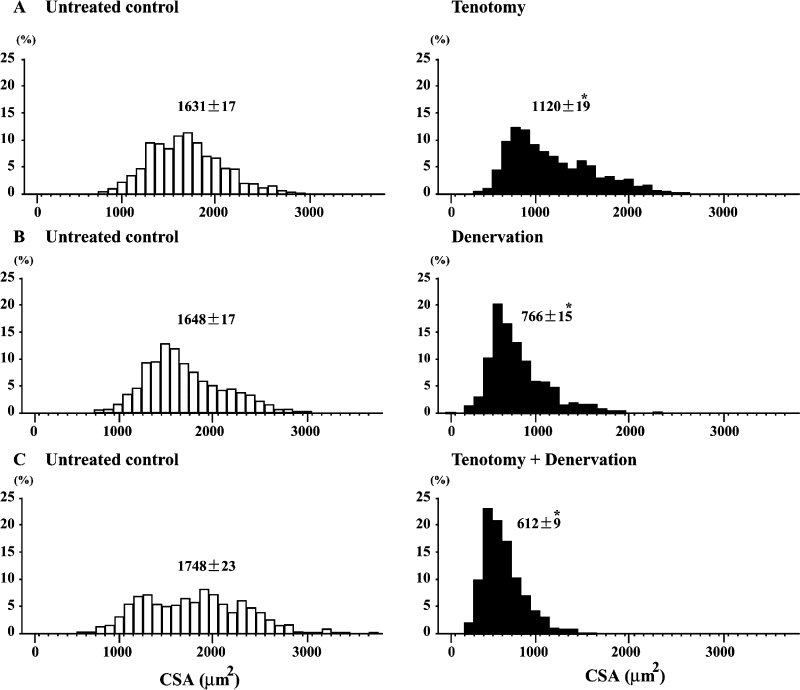
@Fiber CSA. The fiber CSA in the untreated soleus muscle of the wild type
mice ranged between 500 and 3,700 Κm2 (Fig. 1A-C). And the mean
CSA was 1,675 Κm2 (Fig. 3A). It was noted that the fiber CSA became
smaller clearly following T, D, and T+D (p< 0.05). On the contrary, the fiber
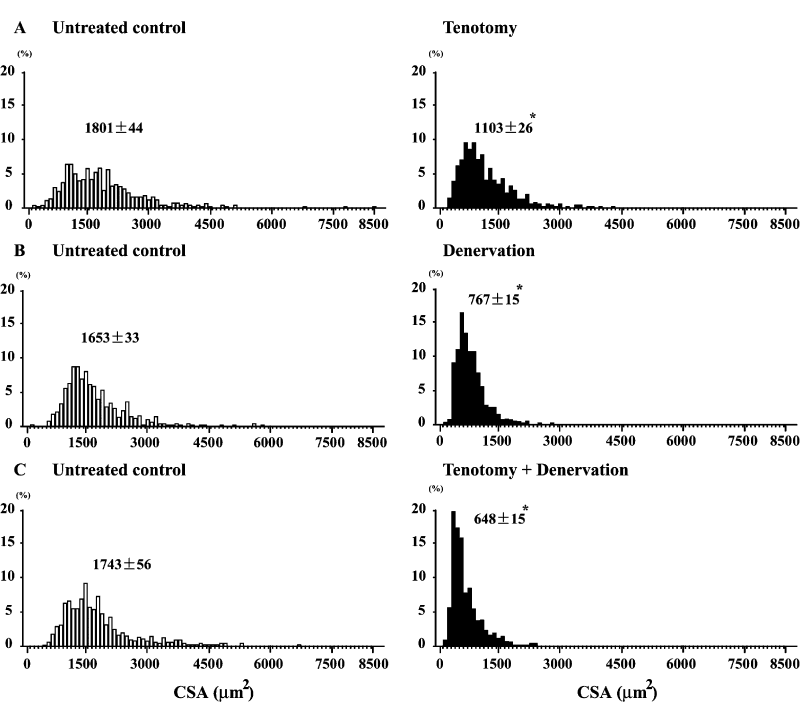
CSA in the untreated control soleus of mdx mice was distributed between 100
to 8,500 Κm2 (Fig. 2A-C) and the mean CSA level was 1,732 Κm2 (Fig.
3A). Larger fibers with more than 4,000 Κm2 of CSA were also noted,
although the number of these fibers was less. The range of the distribution
of fibers with various CSAs became narrower following the treatment with either
T, D, or T+D. And the mean CSA in these muscles treated with T, D, and T+D
was 1,103, 767, and 648 Κm2, respectively, and was significantly
less than the contralateral control side (p< 0.05). But the larger fibers with
CSA of >3,000 Κm2 were still noted in the soleus muscle treated
with T, not with D or T+D.
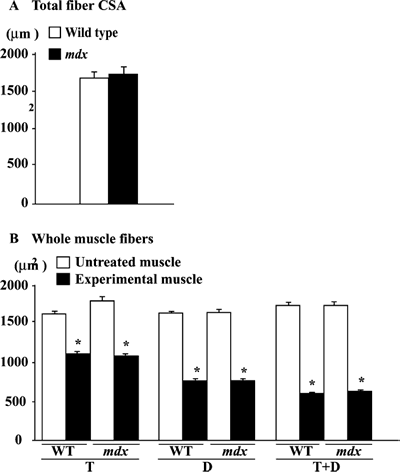
@The mean levels of the fiber CSA in the untreated muscle were identical between
wild type and mdx mice (Fig. 3B). The mean size of the whole muscle fibers
in both types of mice was significantly decreased in response to T, D, or T+D
(p< 0.05). The treatment-related fiber atrophy tended to be advanced as T< D < T
+ D. The fiber CSAs in both mice decreased by `39, 54, and 64% following the
treatment with T, D, and T+D compared with the untreated side, respectively
(p< 0.05). The degree of fiber atrophy was identical between wild type and
mdx mice.
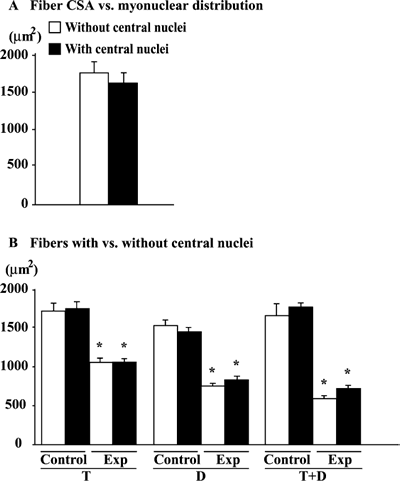
@ The mean fiber CSAs in the untreated muscle without and with central nuclei
was 1,760 and 1,621, respectively (Fig. 4A). The size of the fiber was unrelated
to the localization of myonuclei (Fig. 4B). The fiber CSA in the untreated
muscle was identical between fibers with or without central nuclei. The treatment-related
fiber atrophy was noted. Further, the magnitude of the decrease in the fiber
CSA induced by T, D, or T+D was similar between the fibers with and without
central nuclei.
 |
|
| Fig. 1. | Distribution of fibers with various cross-sectional areas (CSAs) in untreated control and muscle treated with tenotomy and/or denervation in wild type mice. Mean}SEM. *: p<0.05 vs. untreated control, analyzed by ANOVA and Scheffe's post hoc test. |
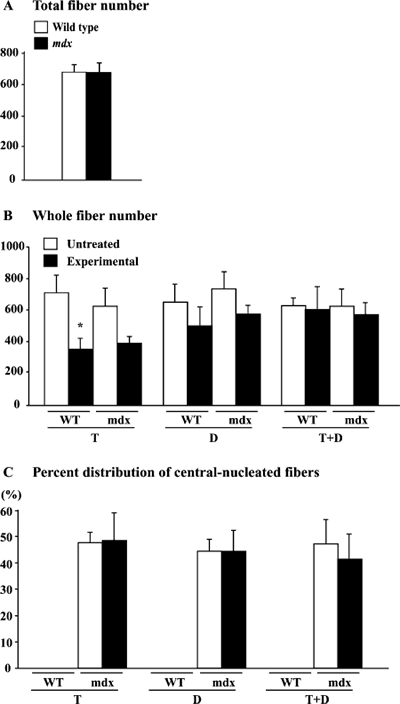
@Total fiber number and the distribution of central nucleated fibers. The
total fiber number in the untreated soleus muscle was `740 in both wild type
and mdx mice (Fig. 5A). The fiber number was decreased by 48.7% (p<0.05)
and 35.3% (p >0.05) in wild type and mdx mice following the application
of T, respectively (Fig. 5B). The fiber number in wild type and mdx mice
was also decreased insignificantly (22.2 and 20.0%) in response to D, respectively
(p >0.05). However, the fiber
number was not affected by T+D. The central nucleated fibers were not seen
in the wild type mice (Fig. 5C). However, the percent distribution of the central
nucleated fibers relative to the total fiber number in mdx mice did
not change in response to any treatments.
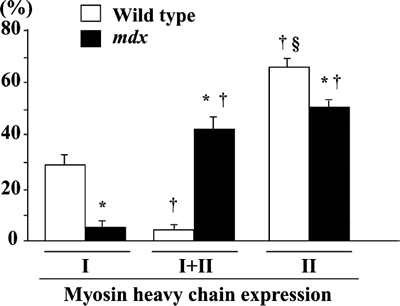
@Fiber phenotype. The mean percent distribution of fibers expressing type
I, I+II, and II MHC in the untreated control muscle was 29.1, 4.7, and 66.2
% in wild type and 5.5, 43.2, and 51.3% in mdx mice (Fig. 6). Significantly
more fibers expressed pure type II MHC fibers than type I+II fibers in wild
type mice (p< 0.05). The percentage of fibers expressing pure type I or II
MHC in untreated mdx mice, including 3 groups, was less than that in wild type
mice (p< 0.05), and the distribution of fibers co-expressing type I+II MHC was
greater than in wild type mice, on the contrary (p< 0.05). No prominent changes
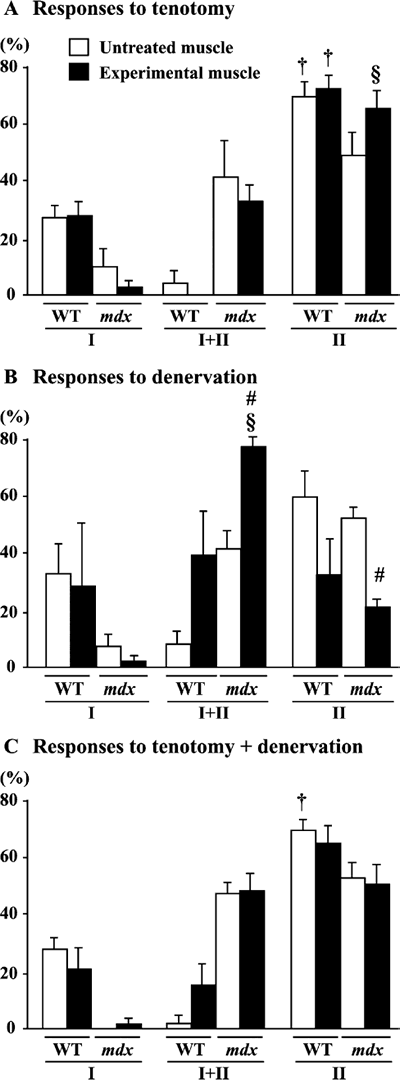
in the distribution of fiber phenotype in wild type mice were observed following
any treatment (Fig. 7A-C). The distribution of the fibers expressing type I+II
MHC in mdx mice was greater (p< 0.05) than that of type I MHC fibers following
D (Fig. 7B). According to the paired comparison in each mouse, the percentage
of type I+II fibers increased (36.0%) and that of type II fibers decreased
(31.1%) significantly in response to D.
 |
|
| Fig. 2. | Distribution of fibers with various cross-sectional areas (CSAs) in untreated control and muscle treated with tenotomy and/or denervation in mdx mice. Mean}SEM. *: p<0.05 vs. untreated control, analyzed by ANOVA and Scheffe's post hoc test. |
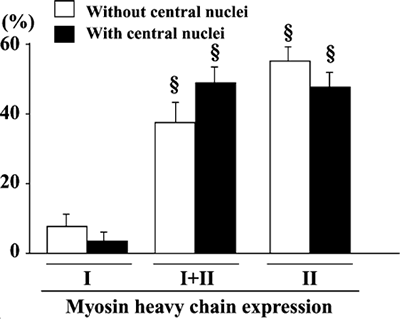
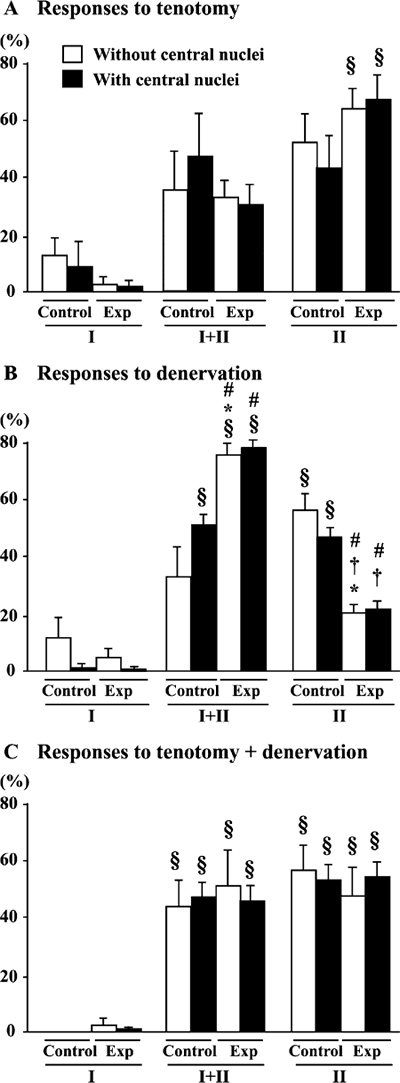
@The relationship between the localization of the myonuclei and the fiber phenotype was also examined in the mdx mice. No significant differences in the fiber phenotype were seen between the untreated control muscle fibers with and without central nuclei (Fig. 8). In response to T or T+D, the percentage of fiber phenotype was not influenced in fibers with and without central nuclei (Fig. 9A and C). However, the percentage of type I+II MHC fibers without central nuclei was increased (130%, p<0.05) and that of type II MHC fibers was decreased significantly (64.7%, p<0.05) following D (Fig. 9B). Similar response was noted in fibers with central nuclei. These changes were statistically significant according to the paired comparison in each mouse. It is suggested that the transformation of fibers, regardless of the localization of myonuclei, were induced from type II to I+II in response to denervation alone. However, such response was not observed, if tenotomy was applied additionally (Fig. 9C).
 |
|
| Fig. 3. | Fiber cross-sectional area in total and whole muscle in untreated control and muscle treated with tenotomy (T) and/or denervation (D) in wild type (WT) and mdx mice (A) and fibers with or without central nuclei in soleus muscle of mdx mice. Mean}SEM. *: p<0.05 vs. untreated control muscle, analyzed by ANOVA and Scheffe's post hoc test. |
 |
|
| Fig. 4. | Fiber cross-sectional area, per myonuclear distribution and with or without central nuclei in soleus muscle of mdx mice. Mean}SEM. *: p<0.05 vs. untreated control muscle, analyzed by ANOVA and Scheffe's post hoc test. Exp: experimental muscle. |
DISCUSSION
@The responses of soleus muscle fibers of mdx and wild type mice to inhibition
of either mechanical loading and/or neural activity were studied. Atrophy of
fibers was induced in response to an inhibition of normal activities, regardless
of dystrophin deficiency or not. The magnitude of the atrophy tended to be
T+D>D>T. Shift of fiber phenotype from pure type II to hybrid type I+II in
mdx, not wild type, mice was induced following D. The degree of both fiber
atrophy and phenotype transformation was identical between the fibers with
or without central nuclei.
@Fiber size : The mean CSA in untreated soleus muscle of wild type mice
was 1,675 Κm2. This was agreed with our previously results (Morikuni
et al., unpublished date). The CSA distribution of untreated soleus muscle
between treatments was different. This might be due to the compensated effect
by any treatments, such as functional over load. The fiber CSA in the untreated
soleus muscle of the wild type mice ranged between 500 and 3,700 Κm2.
On the contrary, extremely small fibers with CSA of 100 Κm2 were
noted in the soleus muscle of mdx mice. Fiber atrophy was induced following
each treatment in both species. Small fibers with CSA of 200-300 Κm2 were
observed in the treated muscle of wild type mice. However, fibers with CSA
less than 100 Κm2 of CSA, which were seen in the untreated muscle,
were not observed in mdx mice, even though the mean CSA level among
the whole muscle fibers decreased `52% in the combined treated muscles. Karpati
et al.16j
reported that small-caliber skeletal muscle fibers in mdx mice do not
suffer necrosis. Even though the total fiber number in both wild type and mdx mice
tended to decreased by T (p >0.05), it was not influenced by D and T+D.
Thus, it is speculated that small fibers with CSA of approximately 100 Κm2 may
not atrophy further in response to inhibition of mechanical load and/or neural
activity.
 |
|
| Fig. 5. | Total fiber number, whole fiber number, and the percent distribution of central nucleated fibers of untreated control or muscle treated with tenotomy (T) and/or denervation (D) in wild type (WT) and mdx mice. Mean}SEM. |
 |
|
| Fig. 6. | Percent distribution of fibers expressing slow (type I) or fast (type II) isoforms of myosin heavy chain. Mean}SEM. and † : p<0.05 vs. type I and type I+II, respectively, analyzed by ANOVA and Scheffe's post hoc test |
@The atrophy of fast-twitch fibers agree with our findings reported that the
CSA of fibers expressing fast-type MHC in rat plantaris muscle decreased in
response to T, D, or T+D associated with hindlimb suspension26j.
The atrophy in plantaris muscle fibers also advanced as T+D>D>T. Therefore,
it is also suggested that the mass of fast-twitch fibers in both rats and mice
may be more influenced by neural activity than on mechanical loading. Further,
the data indicated that myonuclei located at either central or peripheral region
do not play any specific role in the regulation of muscle fiber mass.
@Extremely large fibers were also noted in the soleus muscle of the mdx, not
wild type, mice regardless of the distribution of myonuclei, as was reported
previously20j. It was reported that the following pathways are
activated in mdx mice: the Notch-Delta pathway that plays a role in the activation
of satellite cell and the Bmp15 and Neuregulin 3 signaling pathways that may
regulate the proliferation and differentiation of satellite cells39j.
Schefer et al. was reported that there is no difference in the number of satellite
cells in adult mdx mice and controls32j. However, it was reported
that satellite cells from the dystrophic mdx mouse were more sensitive to fibroblast
growth factor in culture than satellite cells form normal mice7j.
In addition, it was reported that the acceleration of differentiation in satellite
cells from mdx mice, and the ongoing expression of MEF2 in mdx myofiber nuclei
that was especially prominent in the central myonuclei41j. Therefore,
satellite cells from mdx mice may be always more active than wild type mice
and the extreme enlargement of some fibers in mdx mice may be, in part, related
to the excess activation of myoblasts. Such fibers disappeared following D
and T+D, but were still noted in the T group in the present study. This phenomenon
may be related to the loss of the neural innervation in the D and T+D models.
Therefore, it was suggested that the neural innervation might be closely associated
with the production and maintenance of such large fibers in mdx mice.
 |
|
| Fig. 7. | Percent distribution of fibers expressing slow (type I) or fast (type II) isoforms of myosin heavy chain in untreated control and muscle treated with tenotomy and/or denervation in wild type (WT) and mdx mice. Mean}SEM. and † : p<0.05 vs. type I and type I+II, respectively, analyzed by ANOVA and Scheffe's post hoc test. #: p<0.05 vs. untreated control side, analyzed by paired t-test. |
 |
|
| Fig. 8. | Percent distribution of fibers expressing slow (type I) or
fast (type II) isoforms of myosin heavy chain per myonuclear distribution.
: p<0.05 vs. type I, analyzed by ANOVA and Scheffe's post hoc test. |
@Fiber phenotype : The soleus muscle in rat is predominantly composed of slow
fibers (`80-90%)6C8C11C12C28C34j. However, the percentage of type
I fibers in the untreated muscle of wild type mice was only 29.1% in the present
study as was reported elsewhere37C38C40j. The percentage of type
II and I+II fibers were 66.2 and 4.7%, respectively. The percent distribution
of pure type I and II fibers in mdx mice was about 5.5% and 51.3%, respectively,
and significantly less than that of wild type mice. On the contrary, more number
of type I+II fibers (43.2%) was noted in the mdx compared with the wild type
mice (p<0.05). Petrof et al.27j also reported that diaphragm of
mdx mice was composed of more fibers co-expressing type I and IIa MHC, as well
as regenerating fibers expressing embryonic MHC. On the other hand, the percentage
of IIx/b MHC fibers were less than controls. Probably, the muscle in mdx mice
has more regenerating fibers than wild type mice. However, it is not clear
how the expression of MHC is influenced by necrosis/regeneration cycle in mdx
mice.
@Some studies reported that the fiber phenotype of rat soleus muscle was shifted
toward fast type in response to D15C36j. However, there is a discrepancy
in the effects of D on muscle fiber type of mice. Bobinac et al.5j
reported that the D caused the shift of the fiber phenotype toward slow, whereas
other reports suggested that the D induced a shift toward fast type15j.
Further, Bacou et al.2j reported that no effects were observed following
D. The present data showed that the fiber phenotype was shifted from type II
to I+II MHC in response to D, although T and T+D did not affect the phenotype.
Although the precise mechanism responsible for such phenomenon is unclear,
the results suggest that lack of neural activity caused the shift of fibers
from pure type II to hybrid type (I+II). The ankle joint of mice with D was
dorsiflexed, keeping the soleus stretched, when they maintained the quadrupedal
position on the floor. Therefore, some mechanical load was maintained, even
though the neural activity was inhibited. However, D-related effects were inhibited,
if mechanical load was also inhibited by additional treatment with T. The phenotype
transformation in response to D was similar in fibers with myonuclei at either
peripheral or central region, suggesting that myonuclei located at either central
or peripheral region do not play any specific role in the regulation of fiber
phenotype, as well as muscle fiber mass stated above.
 |
|
| Fig. 9. | Percent distribution of fibers, with or without central nuclei in soleus muscle of mdx mice, expressing slow (type I) and/or fast (type II) isoforms of myosin heavy chain in untreated control or muscle (Exp) treated with tenotomy (T) and/or denervation (D). Mean}SEM. *, , and † : p<0.05 vs. untreated control, type I, and type I+II fibers, respectively, analyzed by ANOVA and Scheffe's post hoc test. #: p<0.05 vs. untreated control side, analyzed by paired t-test. |
@Conclusion : Roles of mechanical load and/or neural activity in the regulation
of soleus muscle fibers of wild type and mdx mice were studied. Fiber atrophy
with identical degree in both species was induced in the order of T+D>D>T.
The magnitude of the atrophy was also similar between the fibers with and
without central nuclei. The percentage of fibers expressing pure type I or
II MHC in mdx mice was less than wild type mice, and the distribution of fibers
co-expressing type I and II MHC in mdx mice was greater, on the contrary. The
distribution of type I+II fibers was increased and that of type II fibers decreased
following only D in both types of fibers with myonuclei at either peripheral
or central region in mdx mice. Similar tendency was also observed in wild type
mice, but the changes were not significant statistically. It was suggested
that neural activity and mechanical loading play some roles in the regulation
of the mass and phenotype of soleus muscle fibers in both wild type and mdx
mice. It was further indicated that the regulation of these properties were
not related to the localization of myonuclei.
ACKNOWLEDGEMENTS
@This study was, in part, supported by the Grant-in Aid for Scientific Research
(A, 15200049) from Japan Society for the Promotion of Science, Grant-in-Aid
for Exploratory Research (18650188) from the Ministry of Education, Culture,
Sports, Science and technology, and the Research Grant (16B-2) for Nervous
and Mental Disorders from the Ministry of Health, Labor and Welfare.
References
| 1) | Alford E.K., Roy R.R., Hodgson J.A., Edgerton V.R.: Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hindlimb suspension. Exp Neurol., 96, 635-649, 1987. |
| 2) | Bacou F., Rouanet P., Barjot C., Janmot C., Vigneron P., d'Albis A.: Expression of myosin isoforms in denervated, cross-reinnervated, and electrically stimulated rabbit muscles. Eur J Biochem., 236 (2), 539-547, 1996. |
| 3) | Barton E.R., Morris L., Musaro A., Rosenthal N., Sweeney H.L.: Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol.,157 (1), 137-148, 2002. |
| 4) | Bigard X.A., Janmot C., Merino D., Lienhard F., Guezennec Y.C., D'Albis A.: Endurance training affects myosin heavy chain phenotype in regenerating fast-twitch muscle. J Appl Physiol., 81, 2658-2665, 1996. |
| 5) | Bobinac D., Malnar-Dragojevic D., Bajek S., Soic-Vranic T., Jerkovic R.: Muscle fiber type composition and morphometric properties of denervated rat extensor digitorum longus muscle. Croat Med J., 41 (3), 294-297, 2000. |
| 6) | Choe M.A., An G.J., Lee Y.K., Im J.H., Choi-Kwon S., Heitkemper M.: Effect of inactivity and undernutrition after acute ischemic stroke in a rat hindlimb muscle model. Nurs Res., 53 (5), 283-392, 2004. |
| 7) | Crisona N.J., Allen K.D., Strohman R.C.: Muscle satellite cells from dystrophic (mdx) mice have elevated levels of heparan sulphate proteoglycan receptors for fibroblast growth factor. J Muscle Res Cell Motil., 19 (1), 43-51, 1998. |
| 8) | Desaphy J.F., Pierno S., Liantonio A., De Luca A., Didonna M.P., Frigeri A., Nicchia G.P., Svelto M., Camerino C., Zallone A., Camerino D.C.: Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile. Neurobiol Dis., 18 (2), 356-365, 2005. |
| 9) | Dorchies O.M., Wagner S., Vuadens O., Waldhauser K., Buetler T.M., Kucera P., Ruegg U.T.: Green tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol., 290 (2), C616-C625, 2006. |
| 10) | Haslett J.N., Kang P.B., Han M., Kho A.T., Sanoudou D., Volinski J.M., Beggs A.H., Kohane I.S., Kunkel L.M.: The influence of muscle type and dystrophin deficiency on murine expression profiles. Mamm Genome., 16 (10), 739-748, 2005. |
| 11) | Hodgson J.A., Roy R.R., Higuchi N., Monti R.J., Zhong H., Grossman E., Edgerton V.R.: Does daily activity level determine muscle phenotype ? J Exp Biol., 208 (Pt 19), 3761-3770, 2005. |
| 12) | Ishihara A., Kawano F., Okiura T., Morimatsu F., Ohira Y.: Hyperbaric exposure with high oxygen concentration enhances oxidative capacity of neuromuscular units. Neurosci Res., 52 (2), 146-152, 2005. |
| 13) | Jakubiec-Puka A., Kulesza-Lipka D., Kordowska J.: The contractile apparatus of striated muscle in the course of atrophy and regeneration. II. Myosin and actin filaments in mature rat soleus muscle regenerating after reinnervation. Cell Tissue Res., 227, 641-650, 1982. |
| 14) | Jakubiec-Puka A., Catani C., Carraro U.: Myosin heavy-chain composition in striated muscle after tenotomy. Biochem J., 282, 237-242, 1992. |
| 15) | Jaweed M.M., Herbison G.J., Ditunno J.F.: Denervation and reinnervation of fast and slow muscles. A histochemical study in rats. J Histochem Cytochem., 23 (11), 808-827, 1975. |
| 16) | Karpati G., Carpenter S., Prescott S.: Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy. Muscle Nerve., 11, 795-803, 1988. |
| 17) | Kawano F., Ishihara A., Stevens J.L., Wang X.D., Ohshima S., Horisaka M., Maeda Y., Nonaka I., Ohira Y.: Tension- and afferent-input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am J Physiol; Regul Integr Comp Physiol., 287: R76-R86, 2004. |
| 18) | Kawano F., Nomura T., Ishihara A., Nonaka I., Ohira Y.: Afferent input-associated reduction of muscle activity in microgravity environment. Neuroscience, 114, 1133-1138, 2002. |
| 19) | Kawano F., Wang X.D., Lan Y.B., Yoneshima H., Ishihara A., Igarashi M., Ohira Y.: Hindlimb suspension inhibits air-righting due to altered recruitment of neck and back muscles in rats. Jpn J Physiol., 54, 229-242, 2004. |
| 20) | Lynch G.S., Hinkle R.T., Chamberlain J.S., Brooks S.V., Faulkner J.A.: Force and power output of fast and slow skeletal muscles from mice 6-28 months mdx old. J Physiol., 535, 591-600, 2001. |
| 21) | Marchand E., Constantin B., Balghi H., Claudepierre M.C., Cantereau A., Magaud C., Mouzou A., Raymond G., Braun S., Cognard C.: Improvement of calcium handling and changes in calcium-release properties after mini- or full-length dystrophin forced expression in cultured skeletal myotubes. Exp Cell Res., 297 (2), 363-379, 2004. |
| 22) | Ohira M., Hanada H., Kawano F., Ishihara A., Nonaka I., Ohira Y.: Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn J Physiol., 52, 235-245, 2002. |
| 23) | Ohira Y., Nomura T., Kawano F., Sato Y., Ishihara A., Nonaka I.: Effects of nine weeks of unloading on neuromuscular activities in adult rats. J Gravit Physiol., 9: 49-60, 2002. |
| 24) | Ohira Y., Edgerton V.R.: Neuromuscular adaptation to gravitational unloading or decreased contractile activity. Advanced Exer Sports Physiol., 1, 1-12, 1994. |
| 25) | Ohira Y., Yasui W., Roy R.R., Edgerton V.R.: Effects of muscle length on the response to unloading. Acta Anat., 159, 90-98, 1997. |
| 26) | Ohira Y., Yoshinaga T., Ohara M., Kawano F., Wang X.D., Higo Y., Terada M., Matsuoka Y., Roy R.R., Edgerton V.R.: The role of neural and mechanical influences in maintaining normal fast and slow muscle properties. Cells Tissues Organs, 182, 129-142, 2006. |
| 27) | Petrof B.J., Stedman H.H., Shrager J.B., Eby J., Sweeney H.L., Kelly A.M.: Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol., 265, C834-841, 1993. |
| 28) | Picquet F., Bouet V., Cochon L., Lacour M., Falempin M.: Changes in rat soleus muscle phenotype consecutive to a growth in hypergravity followed by normogravity. Am J Physiol Regul Integr Comp Physiol., 289 (1), R217-R224, 2005. |
| 29) | Riley D.A., Ilyina-Kakueva E.I., Ellis S., Bain J.L.W., Slocum G.R., Sedlak F.R.: Skeletal muscle fiber, nerve, and, blood vessel breakdown in space-flown rats. FASEB J., 4, 84-91, 1990. |
| 30) | Sandri M., Carraro U.: Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol., 31 (12), 1373-1390, 1999. |
| 31) | Sandri M., Minetti C., Pedemonte M., Carraro U.: Apoptotic myonuclei in human Duchenne muscular dystrophy. Lab Inves., 78 (8), 1005-1016, 1998. |
| 32) | Schafer R., Zweyer M., Knauf U., Mundegar R.R., Wernig A.: The ontogeny of soleus muscles in mdx and wild type mice. Neuromuscul Disord., 15(1), 57-64 2005. |
| 33) | Sillitoe R.V., Benson M.A., Blake D.J., Hawkes R.: Abnormal dysbindin expression in cerebellar mossy fiber synapses in the mdx mouse model of Duchenne muscular dystrophy. J Neurosci., 23 (16), 6576-6585, 2003. |
| 34) | Snow L.M., McLoon L.K., Thompson L.V.: Adult and developmental myosin heavy chain isoforms in soleus muscle of aging Fischer Brown Norway rat. Anat Rec A Discov Mol Cell Evol Biol., 286 (1), 866-873, 2005. |
| 35) | Spencer M.J., Walsh C.M., Dorshkind K.A., Rodriguez E.M., Tidball J.G.: Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest., 99 (11), 2745-2751, 1997. |
| 36) | Stevens L., Toursel T., Lenfant A.M., Falempin M., Mounier Y.: Suppression of Motor Innervation Induces Fiber Diversity in Rat Soleus Muscle. Basic Appl Myol., 10 (4), 181-190, 2000. |
| 37) | Talmadge R.J., Otis J.S., Rittler M.R., Garcia N.D., Spencer S.R., Lees S.J., Naya F.J.: Calcineurin activation influences muscle phenotype in a muscle-specific fashion. BMC Cell Biol., 28, 5-28, 2004. |
| 38) | Totsuka Y., Nagao Y., Horii T., Yonekawa H., Imai H., Hatta H., Izaike Y., Tokunaga T., Atomi Y.: Physical performance and soleus muscle fiber composition in wild-derived and laboratory inbred mouse strains. J Appl Physiol., 95 (2), 720-727, 2003. |
| 39) | Turk R., Sterrenburg E., de Meijer E.J., van Ommen G.J., den Dunnen J.T., 't Hoen P.A.: Muscle regeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics, 6, 98, 2005. |
| 40) | Wigston D.J., English A.W.: Fiber-type proportions in mammalian soleus muscle during postnatal development. J Neurobiol 23 (1): 61-70, 1992 |
| 41) | Yablonka-Reuveni Z., Anderson J.E.: Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn., 235(1), 203-212, 2006. |
Send correspondence to :
@Section of Applied Physiology
@Graduate School of Medicine, Osaka University
@Health and Sport Science Research Building
@1-17 Machikaneyama-cho, Toyonaka City, Osaka 560-
@0043, Japan
@Phone and FAX: +81-6-6850-6032
@E-mail: ohira@space.hss.osaka-u.ac.jp