FqσΒ«γw Vol. 44, No. 1, 13-18, 2007
Original
Skeletal Muscle Hypertrophy Induced by Low-Intensity Exercise with Heat-Stress in Healthy Human Subjects
Katsumasa Goto1C2, Hideshi Oda3, Shigeta Morioka2, Toshihito Naito2, Tatsuo Akema2, Haruyasu Kato4, Hiroto Fujiya4, Yasuo Nakajima5, Takao Sugiura6, Yoshinobu Ohira7C8, Toshitada Yoshioka2C9
1Laboratory of Physiology, Toyohashi SOZO University, Toyohashi
2Department of Physiology, St. Marianna University School of Medicine, Kawasaki
3Health Care Products Research 2nd Laboratories, KAO Corporation, Tokyo
4Department of Sports Medicine, St. Marianna University School of Medicine, Kawasaki
5Department of Radiology, St. Marianna University School of Medicine, Kawasaki
6Faculty of Education, Yamaguchi University, Yamaguchi
7Graduate School of Medicine and
8Frontier Biosciences, Osaka University, Toyonaka
9Hirosaki Gakuin University, Hirosaki
ABSTRACT
@Effects of low-intensity exercise (LIE) combined with or without heat-stress
on muscle hypertrophy were investigated. Nine healthy men were subjected to
10 weeks of LIE for elbow flexor muscles{4 days/wk at less than 30 repetition
maximum (RM), 3 sets (30 repetitions) of flexion-extension exercise of elbow
joints}. The 60-min heat-stress was applied to only non-dominant arm, which
performed LIE during the last 30-min period, by using a heat- and steam-generating
sheet (heating area was set for 430 cm2, Kao Corporation, Tokyo,
Japan). The dominant arm was subjected to only LIE. Maximum isometric force
in flexion of the heated non-dominant, not the unheated dominant, arm significantly
increased (18.4%) after 10-week LIE (p< 0.05). However, the maximum isometric
torque in extension of both arms was not improved. Mean cross-sectional area
of biceps brachii muscles in the non-dominant, not the dominant, arm was significantly
increased by LIE combined with heat-stress for 10 weeks (7.5%, p< 0.05). It
was strongly indicated that exercise training at an intensity even lower than
50% 1 RM could be effective in increasing of muscle strength associated with
hypertrophy, when the training was combined with heating. These results suggest
that heat-stress might be a useful countermeasure for prevention of muscular
atrophy during space flight and/or long-term bed rest. Its application could
be also extended to rehabilitation.
(Received: 29 November, 2006 Accepted:
5 December, 2006)
Key words: heating sheet, muscle hypertrophy, low-intensity
exercise, human
INTRODUCTION
@It is well-known that mechanical loading, such as stretch, is one of the primary
factors regulating the synthesis and degradation of proteins in skeletal muscles6C21C31j.
Increased loading on skeletal muscles stimulates protein synthesis and often
results in hypertrophy. In contrast, decreased loading during the exposure
to space or simulated microgravity environment causes a loss of muscular protein
and atrophy19C23j, and then results in a decreased force
generation29j. Therefore, the establishment of a countermeasure
for prevention of muscle atrophy caused by space flight is essential. It is
generally considered that the mechanical stretch is one of the useful tools
as the countermeasure22C24C29j. However,
the muscle atrophy during space flight is not completely prevented by mechanical
stretch as well as heavy resistant exercise. Effective and/or useful countermeasure
for muscle atrophy is not developed yet.
@Recently, it is reported that heat-stress could partially prevent the unloading-related
muscular atrophy19j. Heat-stress stimulates the synthesis of heat
shock protein 72 (HSP72) in muscular cells. We have also shown that the application
of heat-stress at 41 for 60 min could facilitate the stretch-induced muscle
hypertrophy9C30j and loading-induced muscle re-growth7j.
Heat-stress could also induce muscle hypertrophy in cultured skeletal muscle
cells and animal models7C9C13C14C2C30j.
Evidences obtained in our previous studies strongly suggested that the application
of heat-stress may be useful as a countermeasure for muscle atrophy. However,
there is a technical problem that the heating at 41 for 60 min is difficult
to apply for human skeletal muscles.
@In human, it is impossible to increase the body temperature up to 41 by heat-stress.
However, it has been confirmed that heat-stress at `38 for 30-40 min could
induce muscle hypertrophy in rats8j. Therefore, this heating condition
may be applicable to human in order to induce muscle hypertrophy, although there
is no evidence regarding the effects of heat-stress on skeletal muscle in human
subjects. Therefore, this study was performed to investigate the effects of
exercise training at low intensity combined with heat-stress on muscle hypertrophy.
MATERIALS AND METHODS
@Subjects
@Nine healthy men{age: 38.2}5.8 years old (mean}SD), height: 171.8}4.2
cm, body weight: 67.8}5.0 kg}participated in the 10-week study. All experimental
procedures were conducted in accordance with World Medical Association Declaration
of Helsinki (Ethical Principles for Medical Research Involving Human Subjects).
The study was also approved by the Bioethics Committee at St. Marianna University
School of Medicine. All subjects were informed about the possible risks in this
study, and a signed informed consent was obtained from each subject.
@Application of heat-stress
@A heat- and steam-generating sheet (heating area is set for 430 cm2,
Kao Corporation, Tokyo, Japan) was placed on only the non-dominant upper arm,
not the dominant arm. The sheet produces high heat flux and warms the skin more
widely and deeply than the heat-generating sheet without steam20j.
The detail for the heat- and steam-generating sheet is described elsewhere20j.
Briefly, the sheet contains the powder of iron and a small amount of water.
The powder of iron in the sheet reacts with atmospheric oxygen in the air, and
then heat and steam are produced. Each subject also wore supporter on forearm
and gloves in both hands to depress the heat radiation. The heating was applied
for 60 min and exercise was performed during the last 30-min period 4 days a
week for a period of 10 weeks. Skin temperature under the sheet at 30 and 60
min after the initiation of heating was approximately 40.
@Measurement of muscular temperature
@At a depth of 15 mm below the skin surface, the temperature of midbelly region
of biceps brachii muscle of five of nine subjects was measured by using a digital
thermometer (PTC-201, Unique Medical, Tokyo) equipped with a needle thermo-sensor
(TOG203-143B, Unique Medical) immediately before, and 30 and 60 min after the
initiation of heating.
@Low-intensity exercise
@All subjects performed flexion-extension exercise of elbow joint in both the
non-dominant and the dominant arms between 30 and 60 min after the initiation
of heating procedure. The exercise sessions were consisted of 3 sets (30 repetitions)
of exercise against a resistance at less than 30 repetition maximum (RM) with
3 min of rest between each set and were performed 4 days a week for a period
of 10 weeks. Low-intensity exercise (LIE) training was performed by the non-dominant
arm, which was also heated. On the other hand, only LIE was subjected for the
dominant arm.
@Measurements
@The measurements of isometric torque and the cross-sectional muscle size by
computed tomography (CT) were performed in both arms 1 week before the initiation
of study and 3 days after the termination of 10-week training.
@Isometric torque: Isometric torque measurements of both hands were performed
by using Biodex-System (Biodex Medical Systems, Shirley, NY). Subjects performed
a maximum voluntary contraction at 90ί of elbow flexion twice, and then extension
twice. Each contraction was performed for 5 seconds with 5-second interval.
@CT analyses: The CT images were acquired by using Asteion Multi Slice CT (Toshiba
Medical Systems Corporation, Otawara, Tochigi, Japan). This measurement was
done in both upper arms. Four cross-sectional images with 5-mm thick were obtained
at the mid-portion of upper arm with the maximum circumference (100 mm-proximal
position from olecranon, 120 kv and 30 mA). The CT images were used for the
measurement of cross-sectional area (CSA) of biceps brachii muscle. The CSA
was calculated by using SCION image.
@Statistical analyses
@All data were presented as means}SD. Statistical significance was examined
by using one-way ANOVA followed by Scheffe's post hoc test for muscular temperature
and by paired t-test for the maximum isometric torque and CSA. Differences were
considered significant at the 0.05 level of confidence.
RESULTS
@Muscle temperature
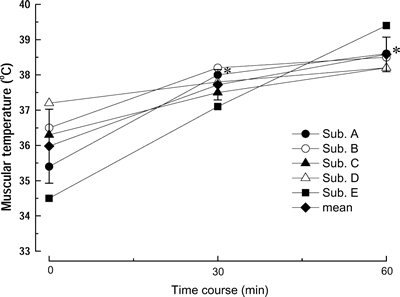
@Figure 1 shows the changes in the temperature of biceps brachii muscles in 5
subjects. The mean muscle temperature increased from `36 to 38 30 min after
the initiation of heating (p<0.05) and was maintained during the remaining
30-min period.
@Maximum isometric torque
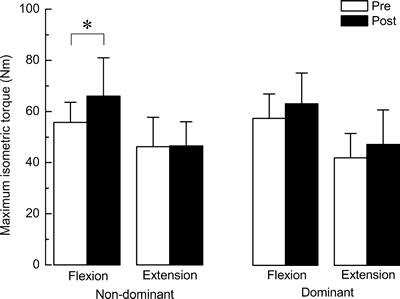
@The mean maximumisometric torque of biceps brachii muscle in nine subjects
is indicated in Figure 2. The mean increase of the maximum isometric torque during
the arm flexion in the heated non-dominant arm was 18.4}11.0% after 10-week LIE
(p<0.05). However, the maximum isometric torque during flexion did
not increase significantly in the unheated dominant arm (11.5}20.5%, p >0.05).
There was no significant increase in the maximum isometric torque during extension
in both heated and unheated arms.
 |
|
| Fig. 1. | Time course changes in the temperature of biceps brachii muscles in 5 subjects during heating procedure. Mean: means}SD of 5 subjects. *: p<0.05 vs. 0 min (before heat exposure). |
 |
|
| Fig. 2. | Mean maximum isometric torque during the flexion and extension of elbow joint. Values are means}SD. n=9 per group. Non-dominant: exercised and heat-stressed non-dominant arm, dominant: exercised dominant arm without heat-stress. Pre: 1 week before the initiation of exercise training, and Post: 3 days after the last bout of 10-wk exercise training. *: p<0.05. |
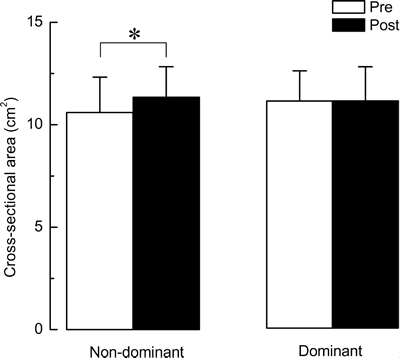
@Cross-sectional area
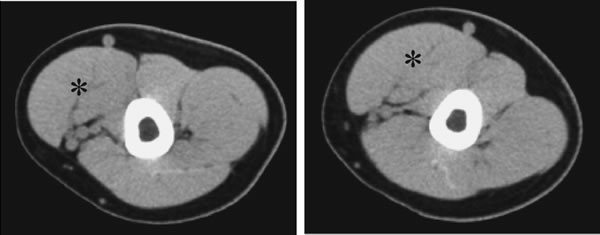
@Figure 3 showed the representative CT images obtained before and after training
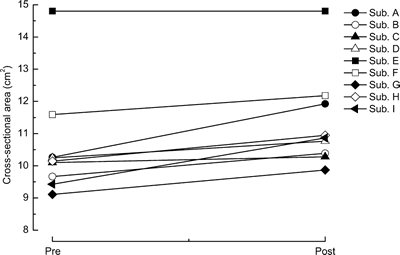
in the same subject. Increase in the CSA of biceps brachii muscle in non-dominant
arm, with both exercise training and heat-stress, was observed in eight out of
nine subjects (Fig. 4). There was no change in one subject, who had the largest
CSA of biceps among subjects. Significant increment of the CSA of biceps brachii
muscles in the heated non-dominant arm was observed following 10-week LIE (Fig.
5, 7.5}5.5%, p<0.05). However, there was no change in the CSA of the
unheated dominant arm (+0.3}4.8%, p >0.05).
 |
|
| Fig. 3. | Representative images of the computed tomography obtained from one subject before and after 10-week low-intensity exercise training combined with heat-stress. Hypertrophy of biceps brachii muscle (*) was observed. Abbreviations are the same as in Fig. 2. |
 |
|
| Fig. 4. | Changes in the cross-sectional area of biceps brachii muscle in each subject in response to the 10 weeks of low-intensity exercise training combined with heat-stress. Abbreviations are the same as in Fig. 2. |
 |
|
| Fig. 5. | Mean cross-sectional area of biceps brachii muscles. Values are means}SD. n=9 per group. Abbreviations are the same as in Fig. 2. *: p<0.05. |
DISCUSSION
@This study clearly showed that LIE combined with heat-stress induced an increase
in the maximum isometric torque and CSA in biceps brachii muscle of human subjects.
Evidences strongly indicated that exercise training at the intensity even less
than 50% 1 RM could be effective in gaining muscular size and strength when
heat-stress is combined. To our knowledge, there is no report regarding the
increase of muscle temperature induced by using a heating and steam-generating
sheet. The present study demonstrated that the temperature of biceps brachii
muscles was increased up to `38 by the heating procedure.
@It is well-known that heat-stress causes an increase in the expression of
HSP724j. The HSP72 expression in cells is also stimulated by ischemia16j,
protein degeneration2j, and/or hypoxia10j. This phenomenon,
so-called the heat shock response, could be induced in most cells. The HSP72
may have some protective roles against cellular stress as molecular chaperons1C18j.
The HSP72 also plays an important role as chaperoning nascent peptides during
translation, in cellular protein transport, and in stability of cellular proteins15j.
It has been reported that muscle hypertrophy was induced in response to heating
at 38 for more than 45 min in rats8j. Further, exercise training
generally causes an increase of core body temperature11j and of
the expression of HSP72 in skeletal muscles3C5C25|27j.
Therefore, it is suggested that a part of the heat-stress-associated muscle
hypertrophy might be dependent on the chaperoning actions of HSP729C14C28j.
@On the other hand, it has been also reported that there was no up-regulating
of HSP72 expression in skeletal muscles with the colonic temperature in rats
at less than 38 8j. It has been generally considered that
the up-regulation of HSP72 in cells is induced, when the body temperature
is elevated by 3-5 9C12j. However, the muscular
temperature might be still less than the threshold for stimulation of HSP72
expression, even if the environmental temperature is elevated to 38
for 60 min. In the present study, there was no information regarding the up-regulation
of HSP72 expression, because it was not investigated. It has been also suggested
that the up-regulation of HSPs may not play the key role for the heat-stress-associated
muscle hypertrophy induced by exposure to 38 8j.
@Effectiveness of LIE in this study disagrees with the established principle
for programming resistance exercise, because it has been generally believed
that an intensity lower than 65% 1 RM is not useful for gaining muscular size
and strength17j. The present study also confirmed that no significant
increases in the maximum isometric torque in flexion and mean CSA of biceps
brachii muscle were induced by LIE alone (Figs. 2 and 5). Muscle size (CSA of
biceps brachii muscle) and strength (maximum isometric torque) were significantly
increased following LIE combined with heat-stress. Hypertrophy of cultured muscle
cells was also observed when both stretch and heat-stress were applied9C30j.
It has been also reported that heat stress-related enhancement of muscle weight
was due to the increased protein contents28j, because there was no
change in the water contents. Heat stress-associated muscle hypertrophy may
also be caused by the increments in the CSA of each muscle fiber28j.
The data suggested that the heat-stress stimulated the exercise-induced intracellular
signaling(s) contributing the protein synthesis.
@Effect of heat-stress or LIE alone on muscular size and strength was not
investigated in the present study. Therefore, it is unclear whether the increases
in the maximum isometric torque and CSA in the trained non-dominant upper
arm were due to LIE and/or heat-stress per se. However, LIE with heat-stress
clearly caused the increase of muscular size and strength. These observations
suggest that heat-stress may enhance the exercise-induced muscular hypertrophy
by activating the protein synthesis.
CONCLUSION
@The LIE combined with heat-stress induced an increase in the maximum isometric
torque and CSA of biceps brachii muscle of human subjects. Evidences obtained
from this study strongly suggest that heat-stress might be a useful countermeasure
for prevention of muscular atrophy during space flight and/or long-term bed rest.
Its application could be also extended to rehabilitation.
ACKNOWLEDGEMENTS
@This study was supported, in part, by Grant-in-Aid for Scientific Research (C,
17500444, KG; C, 15500453, TY; A, 18200042, TY; A, 15200049, YO) from Japan Society
for the Promotion of Science, and the Research Grant (16B-2) for Nervous and
Mental Disorders from the Ministry of Health, Labor and Welfare (YO).
References
| 1) | Beckmann, R.P., Mizzen, L.A. and Welch, W.J.: Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science, 248, 850-854, 1990. |
| 2) | Chiang, H.L., Terlecky, S.R., Plant, C.P. and Dice, J.F.: A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science, 246, 382-385, 1989. |
| 3) | Chilibeck, P.D., Calder, A.W., Sale, D.G. and Webber, C.E.: A comparison of strength and muscle mass increases during resistance training in young women. Eur. J. Appl. Physiol. Occup. Physiol., 77, 170-175, 1988. |
| 4) | De Maio, A.: The heat-shock response. New Horiz., 3, 198-207, 1995. |
| 5) | Desplanches, D., Ecochard, L., Sempore, B., Mayet-Sornay, M.H. and Favier, R.: Skeletal muscle HSP72 response to mechanical unloading: influence of endurance training. Acta Physiol. Scand., 180, 387-394, 2004. |
| 6) | Goldspink, D.F., Garlick, P.J. and McNurlan, M.A.: Protein turnover measured in vivo and in vitro in muscles undergoing compensatory growth and subsequent denervation atrophy. Biochem. J., 20, 89-98, 1983. |
| 7) | Goto, K., Honda, M., Kobayashi, T., Uehara, K., Kojima, A., Akema, T., Sugiura, T., Yamada, S., Ohira, Y. and Yoshioka, T.: Heat stress facilitates the recovery of atrophied soleus muscle in rat. Jpn. J. Physiol., 54, 285-293, 2004. |
| 8) | Goto, K., Kojima, A., Kobayashi, T., Uehara, K., Morioka, S., Naito, T., Akema, T., Sugiura, T., Ohira, Y. and Yoshioka, T.: Heat stress as a countermeasure for prevention of muscle atrophy in microgravity environment. Jpn. J. Aerospace Environ. Med., 42, 51-59, 2005. |
| 9) | Goto, K., Okuyama, R., Sugiyama, H., Honda, M., Kobayashi, T., Uehara, K., Akema, T., Sugiura, T., Yamada, S., Ohira, Y. and Yoshioka, T.: Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. PfluNgers Arch.-Eur. J. Physiol., 447, 247-253, 2003. |
| 10) | Guttman, S.D., Glover, C.V.C., Allis, C.D. and Gorovsky, M.A.: Heat shock, deciliation and release from anoxia induce the synthesis of the starved T. pyriformis. Cell, 22, 299-317, 1980. |
| 11) | Harris, M.B. and Starnes, J.W.: Effects of body temperature during exercise training on myocardial adaptation. Am. J. Physiol. Heart Circ. Physiol., 280, H2271-H2280, 2001. |
| 12) | Kilgore, J.L., Musch, T.I. and Ross, C.R.: Physical activity, muscle, and the HSP70 response. Can. J. Appl. Physiol., 23, 245-260, 1998. |
| 13) | Kobayashi, T., Goto, K., Kojima, A., Akema, T., Uehara, K., Aoki, H., Sugiura, T., Ohira, Y. and Yoshioka, T.: Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem. Biophys. Res. Commun., 331, 1301-1309, 2005. |
| 14) | Kobayashi, T., Uehara, K., Goto, K., Kojima, A., Honda, M., Akema, T., Sugiura, T., Yamada, S., Ohira, Y. and Yoshioka, T.: Muscular hypertrophy is induced by heat stress in rat skeletal muscles. St. Marianna Med. J., 31, 131-138, 2003. |
| 15) | Ku, Z., Yang, J., Menon, V. and Thomason, D.B.: Decreased polysomal HSP-70 may show polypeptide elongation during skeletal muscle atrophy. Am. J. Physiol. Cell Physiol., 268, C1369-C1374, 1995. |
| 16) | Marber, M.S., Mestril, R., Chi, S.H. and Sayan, M.R.: Overexpression of the rat inducible 70 kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J. Clin. Invest., 95, 1446-1456, 1995. |
| 17) | McDonagh, M.J.N. and Davies, C.T.M.: Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur. J. Appl. Physiol., 52, 139-155, 1984. |
| 18) | Morimoto, R.I.: Cells in stress: transcriptional activation of heat shock genes. Science, 259, 1409-1410, 1993. |
| 19) | Naito, H., Powers, S.K., Demirel, H.A., Sugiura, T., Dodd, S.L. and Aoki, J.: Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J. Appl. Physiol., 88, 359-363, 2000. |
| 20) | Oda, H., Igaki, M., Suzuki, A., Tsuchiya, S., Nagashima, K., Iso, S. and Kanosue, K.: Effects of warming the lower back with a heat and steam generating sheet on thermoregulatory responses and sensation. Jpn. J. Biometeor., 43, 43-50, 2006. |
| 21) | Ohira, Y. and Edgerton, V.R.: Neuromuscular adaptation to gravitational unloading or decreased contractile activity. Adv. Exer. Sports Physiol., 1, 1-12, 1994. |
| 22) | Ohira, Y., Yoshinaga, T., Ohara, M., Nonaka, I., Yoshioka, T., Yamashita-Goto, K., Shenkman, B.S., Kozlovskaya, I.B., Roy, R.R. and Edgerton, V.R.: Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol., 87, 1776-1785, 1999. |
| 23) | Ohira, Y.: Neuromuscular adaptation to microgravity environment. Jpn. J. Physiol., 50, 303-314, 2000. |
| 24) | Ohira, Y., Yoshinaga, T., Nonaka, I., Ohara, M., Yoshioka, T., Yamashita-Goto, K., Izumi, R., Yasukawa, K., Sekiguchi, C., Shenkman, B.S. and Kozlovskaya, I.B.: Histochemical responses of human soleus muscle fibers to long-term bedrest with or without countermeasure. Jpn. J. Physiol., 50, 41-47, 2000. |
| 25) | Paul, A.C. and Rosenthal, N.: Different modes of hypertrophy in skeletal muscle fibers. J. Cell Biol., 156, 751-760, 2002. |
| 26) | Puntschart, A., Vogt, M., Wider, H.R., Hoppeler, H. and Billeter R.: Hsp70 expression in human skeletal muscle after exercise. Acta Physiol. Scand., 157, 411-417, 1996. |
| 27) | Skidmore, R., Gutierrez, J.A., Guerriero, V. and Kregel, K.C.: HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol., 268, R92-R97, 1995. |
| 28) | Uehara, K., Goto, K., Kobayashi, T., Kojima, A., Akema, T., Sugiura, T., Yamada, S., Ohira, Y., Yoshioka, T. and Aoki, H.: Heat stress enhances proliferative potential in rat soleus muscle. Jpn. J. Physiol., 54, 263-271, 2004. |
| 29) | Yamashita-Goto, K., Okuyama, R., Honda, M., Kawasaki, K., Fujita, K., Yamada, Y., Nonaka, I., Ohira, Y. and Yoshioka, T.: Maximal and submaximal forces of slow fibers in human soleus after bed rest. J. Appl. Physiol., 91, 417-424, 2001. |
| 30) | Yamashita-Goto, K., Ohira, Y., Okuyama, R., Sugiyama, H., Honda, M., Sugiura, T., Yamada, S., Akema, T. and Yoshioka, T.: Heat stress facilitates stretch-induced hypertrophy of cultured muscle cells. J. Gravit. Physiol., 9, 145-146, 2002. |
| 31) | Vandenburgh, H.H.: Motion into mass: how does tension stimulate muscle growth ? Med. Sci. Sports Exerc., 19, S142-S149, 1987. |
Address for correspondence:
@Katsumasa Goto, Ph.D.
@Laboratory of Physiology, Toyohashi SOZO University
@20-1 Matsushita, Ushikawa, Toyohashi City, Aichi 440-
@8511, Japan